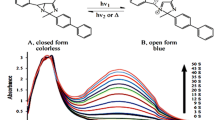
Abstract
We have revisited the photochromic spiro-dihydroindolizine/betaine (DHI/B) system applying state-of-the-art density functional theory (DFT) calculations in combination with stationary and time-resolved absorption measurements. DHI/B-systems are becoming increasingly important as potential molecular machines, molecular switches, and photoswitchable electron-acceptors. The knowledge of the exact mechanisms of ring opening and closure, as well as of the geometries of DHI and betaine can provide critical information that will enable the design of better molecular machines and optical switches. The first surprising result concerns the electronic structure of the betaines, which is quite different than commonly assumed. The photochemical ring opening of DHI’s to betaines is a conrotatory 1,5 electrocyclic reaction, whereas the thermal ring-closing occurs in the disrotatory mode. According to our results, the electrocyclic back reaction of the betaines to the DHI is NOT rate determining, as previously thought, but instead the kinetics are dictated by the cis-trans-isomerization of the betaine.
Similar content being viewed by others
References
G. Hauck and H. Duerr, 1,8a-Dihydroindolizines as components of new photochromic systems, Angewandte Chemie, 1979, 91, 1010–11.
H. Duerr, Perspectives in photochromism:1,5-electrocyclization of pentadienyl anion heteroanalogs as a basis for new systems, Angewandte Chemie, 1989, 101, 427–45.
H. Duerr, H. Gross, K. D. Zils, G. Hauck, G. Klauck and H. Hermann, Photochromic systems. 10. Novel photochromic systems: tetra-, hexa-, and octahydroindolizines, Chemische Berichte, 1983, 116, 3915–25.
S. A. Ahmed, A.-M. A. Abdel-Wahab and H. Durr, Steric substituent effects of new photochromic tetrahydroindolizines leading to tunable photophysical behavior of the colored betaines, J. Photochem. Photobiol. A: Chem, 2003, 154, 131–144.
H. Duerr, C. Schommer and T. Muenzmay, Photochromic systems. 11. Dihydropyrazolopyridine and bis(dihydroindolizines)- new mono- and bifunctional polychromic systems, Angewandte Chemie, 1986, 98, 565–7.
H. Durr, A. Thome, U. Steiner, T. Ulrich, C. Kruger and E. Raabe, Photochromic systems. Part 12. 1H-Benzo[c]pyrazolo[1,2-a]cinnolines. A novel photochromic system, J. Chem. Soc. Chem. Commun., 1988, 338–40.
J. C. Crano, and R. J. Guglielmetti, Organic Photochromic and Thermochromic Compounds Volume 1: Main Photochromic Families, Plenum Press, New York, 1999, pp. 378.
S. D. Straight, J. Andreasson, G. Kodis, A. L. Moore, T. A. Moore and D. Gust, Photochromic Control of Photoinduced Electron Transfer. Molecular Double-Throw Switch, J. Am. Chem. Soc., 2005, 127, 2717–2724.
G. Kodis, Y. Terazono, P. A. Liddell, J. Andreasson, V. Garg, M. Hambourger, T. A. Moore, A. L. Moore and D. Gust, Energy and Photoinduced Electron Transfer in a Wheel-Shaped Artificial Photosynthetic Antenna-Reaction Center Complex, J. Am. Chem. Soc., 2006, 128, 1818–1827.
S. D. Straight, J. Andreasson, G. Kodis, S. Bandyopadhyay, R. H. Mitchell, T. A. Moore, A. L. Moore and D. Gust, Molecular AND and INHIBIT Gates Based on Control of Porphyrin Fluorescence by Photochromes, J. Am. Chem. Soc., 2005, 127, 9403–9409
J. Andreasson, Y. Terazono, B. Albinsson, T. A. Moore, A. L. Moore and D. Gust, Molecular AND logic gate based on electric dichroism of a photochromic dihydroindolizine, Angew. Chem. Int. Ed., 2005, 44, 7591–7594
J. Andreasson, S. D. Straight, G. Kodis, C.-D. Park, M. Hambourger, M. Gervaldo, B. Albinsson, T. Moore, A. L. Moore and D. Gust, All-Photonic Molecular Half-Adder, J. Am. Chem. Soc., 2006, 128, 16259–16265
J. Andreasson, S. D. Straight, S. Bandyopadhyay, R. H. Mitchell, T. A. Moore, A. L. Moore and D. Gust, Molecular 2:1 digital multiplexer, Angew. Chem. Int. Ed., 2007, 46, 958–961
J. Andreasson, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, Molecular All-Photonic Encoder-Decoder, J. Am. Chem. Soc., 2008, 130, 11122–11128.
T. Weitzel, U. Wild, M. Amlung, H. Durr and M. Irie, New photochromic materials for holographic recording, Molecular Crystals and Liquid Crystal Science and Technology, Section A: Molecular Crystals and Liquid Crystals, 2000, 344, 191–198.
J.-F. Masson, T. Hartmann, H. Duerr and K. S. Booksh, Solid-phase synthesis and photochromic switching of a polymeric photochromic layer on a gold surface, Optical Materials (Amsterdam, Netherlands), 2005, 27, 435–439
C. Weber, F. Rustemeyer and H. Duerr, A light-driven switch based on photochromic dihydroindolizines, Advanced Materials, 1998, 10, 1348–1351.
E. Gogritchiani, T. Hartmann, B. S. Palm, S. Samsoniya and H. Durr, Photochromic nucleic base units suitable for nucleic acid labelling, J. Photochem. Photobiol. B: Biol., 2002, 67, 18–22.
H. Duerr, A new photochromic system - potential limitations and perspectives., Pure and Applied Chemistry, 1990, 62, 1477–82.
H. Bouas-Laurent and H. Durr, Organic photochromism, Pure and Applied Chemistry, 2001, 73, 639–665.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, GAUSSIAN 03 (Revision C.02), Gaussian, Inc., Wallingford, CT, 2004.
A. D. Becke, Density-functional exchange-energy approximation with correct asymptotic behavior, Phys. Rev. A, 1988, 38, 3098–100.
C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B, 1988, 37, 785–9.
M. Alajarin, P. Sanchez-Andrada, A. Vidal and F. Tovar, Mode Selectivity in the Intramolecular Cyclization of Ketenimines Bearing N-Acylimino Units: A Computational and Experimental Study, J. Org. Chem., 2005, 70, 1340–1349.
R. Robiette, Mechanism and Diastereoselectivity of Aziridine Formation from Sulfur Ylides and Imines: A Computational Study, J. Org. Chem., 2006, 71, 2726–2734.
Y. Karzazi, G. Vergoten and G. Surpateanu, A density functional theory on the reactivity of disubstituted cycloimmonium ylides, Journal of Molecular Structure, 1998, 417, 83–93.
Y. Karzazi, C. N. Lungu, G. Surpateanu and G. Vergoten, A comparative X-ray diffraction study and ab initio MO calculations on amidocyanopyridinium methylide, Journal of Molecular Structure, 1997, 406, 45–49.
W. Niewodniczanski, W. Bartkowiak and J. Leszcynski, Reinvestigation of molecular structure and barrier to internal rotation pf pyridiniun N-phenolate betaine dye, J. Mol. Model., 2005, 11, 392–397.
R. Robiette, V. K. Aggarwal and J. N. Harvey, Mechanism of the Morita-Baylis-Hillman Reaction: A Computational Investigation, J. Am. Chem. Soc., 2007, 129, 15513–15525.
R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions, J. Chem. Phys., 1980, 72, 650–4.
P. M. Bradley, B. E. Bursten and C. Turro, Excited-State Properties of Rh2(O2CCH3)4(L)2 (L= CH3OH, THF, PPh3, py), Inorg. Chem., 2001, 40, 1376–1379.
J. T. Warren, W. Chen, D. H. Johnston and C. Turro, Ground-State Properties and Excited-State Reactivity of 8-Quinolate Complexes of Ruthenium(II), Inorg. Chem, 1999, 38, 6187–6192.
H. Heydt, Product class 21: diazo compounds, Science of Synthesis, 2004, 27, 843–935.
X. Creary and M. A. Butchko, beta-Silylcarbenes from Isolable Diazosilanes, J. Org. Chem., 2002, 67, 112–118.
F. A. Caroll, Perspectives on Structure and Mechanism in Organic Chemistry, Brooks/Cole Publishing Company, Pacific Grove, Washington, 1998, chapter 11: Concerted Reactions.
http://www.acdlabs.com/products/spec_lab/predict_nmr/cnmr/.
B. H. Besler, K. M. Merz, Jr. and P. A. Kollman, Atomic charges derived from semiempirical methods, J. Comp. Chem., 1990, 11, 431–9.
U. C. Singh and P. A. Kollman, An approach to computing electrostatic charges for molecules., J. Comp. Chem., 1984, 5, 129–45.
J. Cioslowski, A new population analysis based on atomic polar tensors, J. Am. Chem. Soc., 1989, 111, 8333–6.
Y. Sueishi, K. Danjo, S. Yamamoto and N. Nishimura, Kinetic studies of pressure and solvent effects on the thermal isomerization of spiro(1,8-a)dihydroindolizine, Chemistry Express, 1991, 6, 459–62.
H. Gross and H. Duerr, Photochromic system. Part 4. New synthesis of spiro[1,8a]dihydroindolizines; a new photochemically reversible system, Angewandte Chemie, 1982, 94, 204–5.
H. Duerr, and H. Bouas-Laurent, Photochromism: Molecules and Systems, Revised Edition, Elsevier Science B.V, Amsterdam, 2003, pp. 1074.
N. J. Turro, Modern Molecular Photochemistry, Addison-Wesley Publishing Co., Reading, Mass., 1978, pp 628.
H. Bleisinger, P. Scheidhauer, H. Duerr, V. Wintgens, P. Valat and J. Kossanyi, Photophysical properties of biphotochromic dihydroindolizines. Ring-opening into extended bis-betaines, J. Org. Chem., 1998, 63, 990–1000.
R. Fromm, R. Born, H. Durr, J. Kannengiesser, H. D. Breuer, P. Valat and J. Kossanyi, Spirodihydroazafluorenes - a new type of cis-fixed photochromic molecule with rigid region B showing extremely fast back reaction, J. Photochem. Photobiol. A: Chem., 2000, 135, 85–89.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shrestha, T.B., Melin, J., Liu, Y. et al. New insights in the photochromic spiro-dihydroindolizine/betaine-system. Photochem Photobiol Sci 7, 1449–1456 (2008). https://doi.org/10.1039/b814151g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b814151g