Abstract
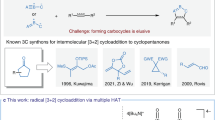
Photoinduced three-component reactions between tetracyanobenzene (TCNB), an alkene and water or a diol (as nucleophile) are initiated by electron transfer from the alkene to 1TCNB* and proceed via a photo-NOCAS reaction sequence by nucleophilic capture of the alkene cation radical and subsequent radical pair coupling of the resulting radical with TCNB anion radical with concomitant cyanide anion extrusion. These photo MCRs provide simple one pot access to isocoumarins and precursors of larger ring lactones with diversified structural features.
Similar content being viewed by others
Notes and references
For reviews, see: J. Zhu, and H. Bienaymé, in Multicomponent Reactions, Wiley-VCH, Weinheim, 2005.
L. F. Tietze, G. Brasche, and K. M. Gericke, in Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006.
A. Dömling, Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry, Chem. Rev., 2006, 106, 17–89.
D. Mangion, D. R. Arnold, Photochemical Nucleophile-Olefin Combination, Aromatic Substitution Reaction. Its Synthetic Development and Mechanistic Exploration, Acc. Chem. Res., 2002, 35, 297–304 and references therein.
I. R. Gould, D. Noukakis, L. Gomes-Jahn, R. H. Young, J. L. Goodman, S. Farid, Radiative and Nonradiative Electron Transfer in Contact Radical-ion Pairs, Chem. Phys., 1993, 176, 439–456.
J. Dresner, J. Prochorow, W. Ode, Kinetics of Weak Molecular Exciplex Formation. Electron Donor-Acceptor Systems of Tetracyanobenzene, J. Phys. Chem., 1989, 93, 671–677.
S. L. Mattes, S. Farid, Photochemical Cycloadditions via Exciplexes, Excited Complexes, and Radical Ions, Acc. Chem. Res., 1982, 15, 80–86.
In our previous study on PET induced olefin dimerization- aromatic substitution reactions between TCNB and alkenes, we also found that the secondary photoreactions between the primary monosubstituted product (5-alkyl-1,2,4-tricyanobenzene) with alkenes, C5 is the site of radical pair coupling with the dimeric alkene cation radical and it was the CN group at C5 that was substituted to give meta-dialkylated products. We have also shown that, this regioselectivity is likely a result of the spin density distribution in the 5-alkyl-1,2,4-tricycnobenzene anion radical (see Z.-F. Lu, Y. Liu, G. Grampp, H.-W. Hu, J.-H. Xu, Photoreactions of 1,2,4,5-Benzenetetracarbonitrile with Arylethenes - Photo-Olefin Dimerization-Aromatic Substitution Reactions, Tetrahedron, 2006, 62, 5663–5674 ref. 5).
M. Zhang, Z.-F. Lu, Y. Liu, G. Grampp, H.-W. Hu, J.-H. Xu, Photoreactions of 1,2,4,5-Benzenetetracarbonitrile with Arylethenes - Photo-Olefin Dimerization-Aromatic Substitution Reactions, Tetrahedron, 2006, 62, 5663–5674.
M. Vanossi, M. Mella, A. Albini, 2 + 2 + 2 Cycloaddition vs. Radical Ion Chemistry in the Photoreactions of 1,2,4,5-Benzenetetracarbonitrile with Alkenes in Acetonitrile, J. Am. Chem. Soc., 1994, 116, 10070–10075.
R. A. Hill, Progress in the Chemistry of Organic Natural Products, Springer-Verlag, Weinheim, 1986, vol. 49, pp. 1–78.
B. V. Mc Inerney, W. C. Taylor, The Xenocoumarins and Related Biologically Active Dihydroisocoumarins, Stud. Nat. Prod. Chem., 1995, 15, 381–422.
Y. Tamaki, A. Furube, M. Murai, K. Hara, R. Katoh, M. Tachiya, Direct Observation of Reactive Trapped Holes in TiO2 Undergoing Photocatalytic Oxidation of Adsorbed Alcohols: Evaluation of the Reaction Rates and Yields, J. Am. Chem. Soc., 2006, 128, 416–417.
X. Cai, M. Sakamoto, M. Fujitsuka, T. Majima, One-Electron Oxidation of Alcohols by the 1,3,5-Trimethoxybenzene Radical Cation in the Excited State during Two-Color Two-Laser Flash Photolysis, J. Phys. Chem. A, 2007, 111, 1788–1791.
Phenylcyclopropane cation radicals are apt to undergo an SN2 nucleophilic attack by ambient nucleophile to give ring-opened benzylic radicals. J. P. Dinnocenzo, T. R. Simpson, H. Zuilhof, W. P. Todd, T. Heinrich, Three-Electron SN2 Reactions of Arylcyclopropane Cation Radicals. 1. Mechanism, J. Am. Chem. Soc., 1997, 119, 987–993.
J. Xue, L. Zhu, H.-K. Fun, J.-H. Xu, Synthesis of Medium and Large Ring Heterocycles by Photoinduced Intermolecular and Intramolecular Electron Transfer Reactions of Tetrachlorophthalimides with Alkenes, Tetrahedron Lett., 2000, 41, 8553–8557.
A. G. Griesbeck, W. Kramer, M. Oelgemöller, New Tools in Organic Synthesis: PET-Decarboxylation: Scope and Limitations, Synlett, 1999, 1169–1178.
A. G. Griesbeck, T. Heinrich, M. Oelgemöller, J. Lex, A. Molis, A Photochemical Route for Efficient Cyclopeptide Formation with a Minimum of Protection and Activation Chemistry, J. Am. Chem. Soc., 2002, 124, 10972–10973.
Crystal structures of 5, 7 and 17 have been determined. Y.-G. Wang, H.-Y. An, Z.-F. Lu, L. Wu, J.-H. Xu, 5-Benzylbenzene-1,2,4-tricarbonitrile, Acta Crystallogr. Sect. E: Struct. Rep. Online, 2005, 61, 3543–3544.
Z.-F. Lu, J.-H. Xu, J. B.-J. Teh, H.-K. Fun, 5-[(4-Methylphenyl)methyl]benzene-1,2,4-tricarbonitrile, Acta Crystallogr. Sect. E: Struct. Rep. Online, 2007, 63, o1230–o1231.
Z.-F. Lu, J.-H. Xu, J. B.-J. Teh, H.-K. Fun, 4,6-Bis2’-hydroxy-2’,3’-dihydrospiro[cyclopropane-1,1’(1H)-inden]-3’-ylisophthalonitrile, Acta Crystallogr. Sect. E: Struct. Rep. Online, 2007, 63, 1232–1234.
Author information
Authors and Affiliations
Corresponding author
Additional information
CCDC reference numbers 683621. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b809550g
Rights and permissions
About this article
Cite this article
Lu, ZF., Yue, JJ., Zhu, Y. et al. Photo-multicomponent reactions leading to the construction of isocoumarins and large ring lactone precursors. Photochem Photobiol Sci 8, 217–223 (2009). https://doi.org/10.1039/b809550g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b809550g