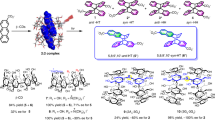
Abstract
Resorc[4]arenes have been difunctionalized on opposite sides of the macrocycle using two anthracene units, which undergo intramolecular dimerization on irradiation at 350 nm. Syntheses and time-dependent photochemical experiments are reported.
Similar content being viewed by others
Notes and references
P. Timmerman W. Verboom D. N. Reinhoudt Resorcinarenes Tetrahedron 1996 52 2663–2704
T. Tsudera A. Ikeda S. Shinkai Light-switched metal-tunneling across a p-basic tube of 1,3-alternate-calix[4]arenes Tetrahedron 1997 53 13609–13620
H. D. Becker Unimolecular photochemistry of anthracenes Chem. Rev. 1993 93 145–172
H. Bouas-Laurent A. Castellan J. P. Desvergne R. Lapouyade Photodimerization of anthracenes in fluid solution: structural aspects Chem. Soc. Rev. 2000 29 43–55
H. Bouas-Laurent A. Castellan J. P. Desvergne R. Lapouyade Photodimerization of anthracenes in fluid solutions: (part 2) mechanistic aspects of the photocycloaddition and of the photochemical and thermal cleavage Chem. Soc. Rev. 2001 30 248–263
J. L. Irwin M. S. Sherburn Practical synthesis of selectively functionalized cavitands J. Org. Chem. 2000 65 602–605
W. H. Pirkle J. M. Finn Useful routes to 9-anthryl ethers and sulfides J. Org. Chem. 1983 48 2779–2780
6: 1H NMR (500 MHz, CDCl3): δ [ppm] = 8.38 (4H, d, J= 8.2 Hz, ArH), 8.14 (2H, s, ArH), 7.91 (4H, d, J= 8.8 Hz, ArH), 7.37 (8H, m, ArH), 7.01 (2H, s, ArH), 6.57 (2H, s, ArH), 6.17 (2H, s, ArH), 4.78 (4H, s, ArCH2), 4.52 (4H, dd, J= 8.5 Hz, J= 6.0 Hz, ArCHAr), 4.38 (4H, t, J= 4.4 Hz, CH2O), 4.10 (4H, t, J= 4.1 Hz, CH2O), 3.93 (12H, s, ArOCH3), 3.43 (12H, s, ArOCH3), 1.72–1.98 (8H, m, CH2), 1.15–1.45 (72H, m, CH2), 0.85 (12H, t, J= 7.2 Hz, CH3). ESI–HRMS: m/z calc. for C114H156O12Na: 1740.14890 ([M + Na]+); found: 1740.15045
7: 1H NMR (500 MHz, CD2Cl2): δ [ppm] = 8.44 (2H, s, ArH), 8.33 (4H, d, J= 8.8 Hz, ArH), 7.98 (4H, d, J= 8.8 Hz, ArH), 7.51 (4H, dd, J= 8.2 Hz, J= 6.9 Hz, ArH), 7.46 (4H, dd, J= 8.2 Hz, J= 6.9 Hz, ArH), 6.76 (2H, s, ArH), 6.74 (2H, s, ArH), 6.30 (2H, s, ArH), 5.41 (4H, s, ArCH2), 4.59 (4H, s, ArCH2), 4.47 (4H, t, J= 7.5 Hz, ArCHAr), 3.58 (12H, s, ArOCH3), 3.57 (12H, s, ArOCH3), 1.82 (4H, m, CH2), 1.75 (4H, m, CH2), 1.17–1.36 (72H, m, CH2), 0.87 (12H, t, J= 6.9 Hz, CH3). ESI–HRMS: m/z calc. for C112H152O10Na: 1680.12849 ([M + Na]+); found: 1680.12904
8: 1H NMR (500 MHz, CD2Cl2): δ [ppm] = 7.50 (2H, s, ArH), 6.94 (4H, d, J= 6.9 Hz, ArH), 6.73–6.82 (12H, m, ArH), 6.62 (2H, s, ArH), 6.59 (2H, s, ArH), 4.71 (4H, t, J= 7.5 Hz, ArCHAr), 4.56 (4H, s, ArCH2), 4.37 (2H, s, CH), 4.03 (12H, s, ArOCH3), 3.69 (4H, s, OCH2), 3.49 (12H, s, ArOCH3), 2.01 (4H, m, CH2), 1.81 (4H, m, CH2), 1.17–1.46 (72H, m, CH2), 0.88 (12H, t, J= 6.9 Hz, CH3)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schäfer, C., Mattay, J. Preparation of photocyclizable dianthracene derivatives of resorc[4]arenes which are potential photoswitches. Photochem Photobiol Sci 3, 331–333 (2004). https://doi.org/10.1039/b400351a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b400351a