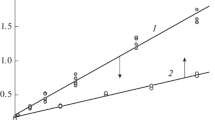
Abstract
A temperature-sensitive photochemical nucleophilic aromatic substitution on 4-nitroanisole by a hydroxide ion in homogeneous solutions, in a two-phase system under phase-transfer catalysis conditions, and in the microwave field is reported. It was found that reaction regioselectivity dramatically changes with temperature in the region of −20 to 196 °C. The quantum yield of the 4-methoxyphenol formation was found to be temperature independent, in contrast to that of the 4-nitrophenol formation, suggesting that there is a temperature dependent process occurring after the partitioning between replacement of the nitro group and the methoxy group has taken place. The reaction was also investigated by using quantum chemical calculations. A technique for microwave-assisted photochemical synthesis is proposed as an efficient and practical tool for organic synthesis.
Similar content being viewed by others
References
J. Cornelisse, in CRC Handbook of Organic Photochemistry, Photobiology, eds W. M. Horpool and P.-S. Song, CRC Press, Boca Raton, 1995, p. 250–265.
J. Cornelisse, G. Lodder, E. Havinga, Pathways and intermediates of nucleophilic aromatic photosubstitution reactions, Rev. Chem. Intermed., 1979, 2, 231–265.
J. Cornelisse, E. Havinga, Photosubstitution reactions of aromatic compounds, Chem. Rev., 1975, 75, 353–388.
C. Parkanyi, Aromatic photosubstitutions, Pure. Appl. Chem., 1983, 55, 331–341.
S. Nilsson, Direct cyanation of aromatic compounds. II. Comparison of isomer distributions from different cyanation methods, Acta. Chem. Scand., 1973, 27, 329–335.
J. Den Heijer, O. B. Shadid, J. Cornelisse, E. Havinga, Photoreactions of aromatic compounds. XXXV. Nucleophilic photosubstitution of methoxy substituted aromatic compounds. Monophotonic ionization of the triplet, Tetrahedron, 1977, 33, 779–786.
H. C. H. A. van Riel, G. Lodder, E. Havinga, Photochemical methoxide exchange in some nitromethoxybenzenes–the role of the nitro-group in SN2Ar* reactions, J. Am. Chem. Soc., 1981, 103, 7257–7262.
E. Havinga, J. Cornelisse, Aromatic photosubstitution reactions, Pure Appl. Chem., 1976, 47, 1–10.
E. Havinga, R. O. de Jongh, Photochemical reactions of nitrophenyl esters and ethers, Bull. Soc. Chim. Belg., 1962, 71, 803–810.
R. L. Letsinger, O. B. Ramsey, J. H. McCain, Photoinduced substitution. II. Substituent effects in nucleophilic displacement on substituted nitrobenzenes, J. Am. Chem. Soc., 1965, 87, 2945–2953.
S. de Vries, E. Havinga, Photoreactions of aromatic compounds. V. Products isolated from the irradiation of mixtures of p-nitroanisole and allyl p-nitrophenyl ether in 0.1N NaOH, Recl. Trav. Chim. Pays-Bas, 1965, 84, 601–602.
M. Sawaura, T. Mukai, Organic-photochemistry. 50 photochemical nucleophilic-substitution reactions of methyl-substituted derivatives of para-nitroanisole and ortho-nitroanisole, Bull. Chem. Soc. Jpn., 1981, 54, 3213–3214.
P. T. Anastas and J. C. Warner, In Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, p. 30.
C. M. Starks,, C. L. Liotta, and M. Halpern, In Phase-transfer Catalysis: Fundamentals, Applications, and Industrial Perspectives, Chapman and Hall, New York, 1994.
A. Guarini, P. Tundo, Rose-bengal functionalized phase-transfer catalysts promoting photooxidations with singlet oxygen-nucleophilic displacements on dioxetanic and endoperoxidic intermediates, J. Org. Chem., 1987, 52, 3501–3508.
J. J. Brunet, C. Sidot, P. Caubere, Sunlamp-irradiated phase-transfer catalysis. 1. Cobalt carbonyl catalyzed SRN1 carbonylations of aryl and vinyl halides, J. Org. Chem., 1983, 48, 1166–1171.
S. Shimada, K. Nakagawa, K. Tabuchi, Photopolymerization of methyl-methacrylate with 1-benzyl-1,4-dihydronicotinamide in the presence of carbon-tetrachloride, Polym. J., 1989, 21, 275–279.
S. Shimada, Y. Obata, K. Nakagawa, K. Tabuchi, Photopolymerization of methyl-methacrylate with methyl viologen-Na2S2O4-CCl4 in aqueous-organic, 2 phase system, Polym. J., 1990, 22, 777–780.
R. Maidan, I. Willner, Photochemical and chemical enzyme catalyzed debromination of meso-1,2-dibromostilbene in multiphase systems, J. Am. Chem. Soc., 1986, 108, 1080–1082.
P. Klán, J. Literák, M. Hájek, The electrodeless discharge lamp: a prospective tool for photochemistry, J. Photochem. Photobiol. A, 1999, 128, 145–149.
J. Literák, P. Klán, The electrodeless discharge lamp: a prospective tool for photochemistry–Part 2. Scope and limitation, J. Photochem. Photobiol. A, 2000, 137, 29–35.
P. Klán, M. Hájek, V. Církva, The electrodeless discharge lamp: a prospective tool for photochemistry Part 3. The microwave photochemistry reactor, J. Photochem. Photobiol. A, 2001, 140, 185–189.
P. Klán, J. Literák, S. Relich, Molecular photochemical thermometers: investigation of microwave superheating effects by temperature dependent photochemical processes, J. Photochem. Photobiol. A, 2001, 143, 49–57.
S. Chemat, A. Aouabed, P. V. Bartels, D. C. Esveld, F. Chemat, An original microwave-ultra violet combined reactor suitable for organic synthesis and degradation, Journal of Microwave Power and Electromagnetic Energy, 1999, 34, 55–60.
The effective power of the oven in the position of the reaction vessel was experimentally found to be approximately 300 W according to: K. W. Watkins, Heating in microwave-ovens–an example of dipole-moments in action, J. Chem. Educ., 1983, 60, 1043.
S. Caddick, Microwave-assisted organic-reactions, Tetrahedron, 1995, 51, 10403–10432.
R. A. Abramovitch, Applications of microwave-energy in organic-chemistry–a review, Org. Prep. Proced. Int., 1991, 23, 685–711.
D. M. P. Mingos, D. R. Baghurst, Applications of microwave dielectric heating effects to synthetic problems in chemistry, Chem. Soc. Rev., 1991, 20, 1–47.
D. R. Baghurst, D. M. P. Mingos, Superheating effects associated with microwave dielectric heating, J. Chem. Soc., Chem. Commun., 1992, 674–677.
C. A. G. O. Varma, J. J. Tamminga, J. Cornelisse, Mechanistic and kinetic aspects of the photoinduced OCH3 substitution in 3,5-dinitroanisole–a probe for solvent effects in thermal-reactions, J. Chem. Soc., Faraday Trans., 1982, 78, 265–284.
P. H. M. Van Zeijl, L. M. J. van Eijk, C. A. G. O. Varma, Spectroscopic and kinetic-study of the photoinduced methoxy substitution of 3-nitroanisole and 3,5-dinitroanisole, J. Photochem., 1985, 29, 415–433.
K. Mutai, R. Nakagaki, H. Tukada, Photoinduced intramolecular substitution. 4. a rationalization of orientation in nucleophilic aromatic photosubstitution, Bull. Chem. Soc. Jpn., 1985, 58, 2066–2071.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle and J. A. Pople, GAUSSIAN 98 (Revision A.9), Gaussian, Inc., Pittsburgh, PA, 1998.
S. D. Naik, L. K. Doraiswamy, Phase transfer catalysis: Chemistry and engineering, AIChE J., 1998, 44, 612–646.
V. Dragojlovic, D. Bin Gao, Y. L. J. Chow, Multigram scale cobalt catalyzed photochemical methoxycarbonylation of alkenes, J. Mol. Catal. A: Chem., 2001, 171, 43–51.
R. Beugelmans, H. Ginsburg, A. Lecas, M.-T. Le Goff, G. Roussi, Use of phase transfer agents for photocyanation of aromatic hydrocarbons, Tetrahedron Lett., 1978, 35, 3271–3274.
R. Beugelmans, H. Ginsburg, A. Lecas, M.-T. Le Goff, J. Pusset, G. Roussi, Use of tetrabutylammonium cyanide for photocyanation of aromatic compounds: phase transfer photochemistry, J. Chem. Soc., Chem. Commun., 1977, 23, 885–886.
R. Růžička, M. Zabadal, P. Klán, Photolysis of phenacyl esters in a two-phase systém, Synth. Commun., 2002, 32, 2581–2590.
S. A. Galema, Microwave chemistry, Chem. Soc. Rev., 1997, 26, 233–238.
R. Dagani, Molecular magic with microwaves, Chem. Eng., 1997, 75, 26–33.
L. Perreux, A. Loupy, A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations, Tetrahedron, 2001, 57, 9199–9223.
P. Klán, and V. Církva, Microwave Photochemistry, in: Microwaves in Organic Synthesis, ed. A. Loupy, Wiley-VCH, 2002.
E. Den Besten, J. W. Tracy, Electrodelessly discharged photochemical lamps, J. Chem. Edu., 1973, 50, 303–303.
V. Církva, M. Hájek, Microwave photochemistry. Photoinitiated radical addition of tetrahydrofuran to perfluorohexylethene under microwave irradiation, J. Photochem. Photobiol. A, 1999, 123, 21–23.
F. Chemat, E. Esveld, Microwave super-heated boiling of organic liquids: Origin, effect and application, Chem. Eng. Technol., 2001, 24, 735–744.
R. Saillard, M. Poux, J. Berlan, M. Audhuy-Peaudecerf, Microwave-heating of organic-solvents–thermal effects and field modeling, Tetrahedron, 1995, 51, 4033–4042.
A. Stadler, C. O. Kappe, The effect of microwave irradiation on carbodiimide-mediated esterifications on solid support, Tetrahedron, 2001, 57, 3915–3920.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klán, P., Růžička, R., Heger, D. et al. Temperature-sensitive photochemical aromatic substitution on 4-nitroanisole. Photochem Photobiol Sci 1, 1012–1016 (2002). https://doi.org/10.1039/b209010d
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b209010d