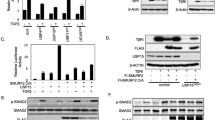
Abstract
Disruption of components in the transforming growth factor-β (TGF-β) signalling cascade is a common occurrence in human cancers. TGF-β pathway activation is accomplished via serine/threonine kinase receptors and intracellular Smad transcription factors. A key regulatory step involves specific ubiquitination by Smurfs that mediate the proteasomal degradation of Smads and/or receptors. Here, we report a novel interaction between Smads and ubiquitin C-terminal hydrolase UCH37, a deubiquitinating enzyme that could potentially reverse Smurf-mediated ubiquitination. In GST pull down experiments, UCH37 bound weakly to Smad2 and Smad3 and bound very strongly to Smad7 in a region that is distinct from the –PY– motif in Smad7 that interacts with Smurf ubiquitin ligases. Endogenous Smad7 and UCH37 formed a stable complex in U4A/JAK1 cells and FLAG-Smad7 co-immunoprecipitated with HA-UCH37 in transfected HEK-293 cells. In addition, we show that UCH37 can deubiquitinate and stabilize the type I TGF-β receptor. Furthermore, overexpression of UCH37 upregulates TGF-β-dependent transcription and this effect is reversed in cells subject to RNAi-mediated knockdown of endogenous UCH37. These findings support a new role for deubiquitinating enzymes in the control of the TGF-β signalling pathway and provide a novel molecular target for the design of inhibitors with therapeutic potential in cancer.



Similar content being viewed by others
References
Chantry A . (1995). J. Biol. Chem., 270, 3068–3073.
Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T and Miyazono K . (2001). J. Biol. Chem., 276, 12477–12480.
Glickman MH and Ciechanover A . (2002). Physiol. Rev., 82, 373–428.
Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz DC, Wilkinson KD, Maul GG, Barlev N, Berger SL, Prendergast GC and Rauscher III FJ . (1998). Oncogene, 16, 1097–1112.
Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH and Wrana JL . (2000). Mol. Cell., 6, 1365–1375.
Lam YA, DeMartino GN, Pickart CM and Cohen RE . (1997a). J. Biol. Chem., 272, 28438–28446.
Lam YA, Xu W, DeMartino GN and Cohen RE . (1997b). Nature, 385, 737–740.
Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J and Gu W . (2002). Nature, 416, 648–653.
Lo RS and Massague J . (1999). Nat. Cell Biol., 1, 472–478.
Shi Y and Massague J . (2003). Cell, 113, 685–700.
Suzuki C, Murakami G, Fukuchi M, Shimanuki T, Shikauchi Y, Imamura T and Miyazono K . (2002). J. Biol. Chem., 277, 39919–39925.
Ulloa L, Doody J and Massague J . (1999). Nature, 397, 710–713.
Wicks SJ, Lui S, Abdel-Wahab N, Mason RM and Chantry A . (2000). Mol. Cell. Biol., 20, 8103–8111.
Wing SS . (2003). Int. J. Biochem. Cell Biol., 35, 590–605.
Zhu H, Kavsak P, Abdollah S, Wrana JL and Thomsen GH . (1999). Nature, 400, 687–693.
Acknowledgements
This study was supported by research grants from the BBSRC (AC), Wellcome Trust, UK (AC), NIH Grant R01 GM37666 (REC) and the Dutch Cancer Society (PTD). We thank Caroline Hill, Takeshi Imamura, Ian Kerr and Ana Costa-Pereira for vectors and cell lines. We also thank the Nederlands Kanker Instituut (NKI) and Cancer Research UK for allowing us to use the RNAi-UCH37 construct.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wicks, S., Haros, K., Maillard, M. et al. The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGF-β signalling. Oncogene 24, 8080–8084 (2005). https://doi.org/10.1038/sj.onc.1208944
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208944
- Springer Nature Limited
Keywords
This article is cited by
-
OTUD4 enhances TGFβ signalling through regulation of the TGFβ receptor complex
Scientific Reports (2020)
-
Ubiquitin–proteasome system (UPS) as a target for anticancer treatment
Archives of Pharmacal Research (2020)
-
Proteasomal cysteine deubiquitinase inhibitor b-AP15 suppresses migration and induces apoptosis in diffuse large B cell lymphoma
Journal of Experimental & Clinical Cancer Research (2019)
-
The role of proteases in epithelial-to-mesenchymal cell transitions in cancer
Cancer and Metastasis Reviews (2019)
-
TGF-β signaling pathway mediated by deubiquitinating enzymes
Cellular and Molecular Life Sciences (2019)