Abstract
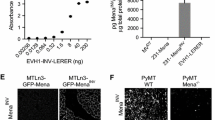
Many mouse models of breast cancer form large primary tumors that rarely metastasize. Models with aggressive metastasis express oncoproteins that simultaneously affect growth and apoptosis pathways. To define the role of apoptotic resistance and to model a challenge faced by tumor cells during metastatic dissemination, we focused on apoptosis induced by cell shape change. Inhibiting actin polymerization with Latrunculin-A causes cell rounding and death within hours in nontumorigenic human 10A-Ras mammary epithelial cells. In contrast, MDA-MB-231 metastatic breast tumor cells resist LA-induced death, and survive for days despite cell rounding. Infecting 10A-Ras cells with a MDA-MB-231 retroviral expression library, and selecting with Latrunculin-A repeatedly identified Bcl-xL as a suppressor of cytoskeleton-dependent death. Although Bcl-xL enhances the spread of metastatic breast tumor cell lines, the distinct effects of apoptotic resistance on tumor growth in the mammary gland and during metastasis have not been compared directly. We find that Bcl-xL overexpression in mouse mammary epithelial cells does not induce primary tumor formation or enhance MEK-induced tumorigenesis within the mammary gland environment. However, it strongly enhances metastatic potential. These results with Bcl-xL provide novel evidence that isolated apoptotic resistance can increase metastatic potential, but remain overlooked by assays based on breast tumor growth.




Similar content being viewed by others
References
Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P and Drubin DG . (1997). J. Cell. Biol., 137, 399–416.
Ben-Ze′ev A . (1997). Curr. Opin. Cell Biol., 9, 99–108.
Birchmeier C, Birchmeier W and Brand-Saberi B . (1996). Acta Anat., 156, 217–226.
Chambers AF, Groom AC and MacDonald IC . (2002). Nat. Rev. Cancer, 2, 563–572.
Chen CS, Mrksich M, Huang S, Whitesides GM and Ingber DE . (1997). Science, 276, 1425–1428.
Eischen CM, Rehg JE, Korsmeyer SJ and Cleveland JL . (2002). Cancer Res., 62, 2184–2191.
Fernandez Y, Gu B, Martinez A, Torregrosa A and Sierra A . (2002). Int. J. Cancer, 101, 317–326.
Frisch SM and Francis H . (1994). J. Cell. Biol., 124, 619–626.
Fujita M, Ichinose S, Kiyono T, Tsurumi T and Omori A . (2003). Oncogene, 22, 627–631.
Furth PA, Bar-Peled U, Li M, Lewis A, Laucirica R, Jager R, Weiher H and Russell RG . (1999). Oncogene, 18, 6589–6596.
Greider C, Chattopadhyay A, Parkhurst C and Yang E . (2002). Oncogene, 21, 7765–7775.
Guy CT, Cardiff RD and Muller WJ . (1992). Mol. Cell. Biol., 12, 954–961.
Jager R, Herzer U, Schenkel J and Weiher H . (1997). Oncogene, 15, 1787–1795.
Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR and Thompson CB . (2000). Mol. Cell, 6, 1389–1399.
Martin SS and Leder P . (2001). Mol. Cell. Biol., 21, 6529–6536.
Martin SS and Vuori K . (2004). BBA Molecular Cell Research, (in press).
Murphy KL, Kittrell FS, Gay JP, Jager R, Medina D and Rosen JM . (1999). Oncogene, 18, 6597–6604.
Nikiforov MA, Kwek SS, Mehta R, Artwohl JE, Lowe SW, Gupta TD, Deichman GI and Gudkov AV . (1997). Oncogene, 15, 3007–3012.
O'Reilly LA, Huang DC and Strasser A . (1996). EMBO J., 15, 6979–6990.
Olopade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB and Recant WM . (1997). Cancer J. Sci. Am., 3, 230–237.
Pelengaris S, Khan M and Evan GI . (2002). Cell, 109, 321–334.
Pinkas J and Leder P . (2002). Cancer Res., 62, 4781–4790.
Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK and Brugge JS . (2003). Nat. Cell Biol., 5, 733–740.
Rubio N, Espana L, Fernandez Y, Blanco J and Sierra A . (2001). Lab. Invest., 81, 725–734.
Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt H, Muller P, Bischoff J, Harich D, Schlimok G, Riethmuller G, Eils R and Klein CA . (2003). Proc. Natl. Acad. Sci. USA, 100, 7737–7742.
Shi HY, Zhang W, Liang R, Kittrell F, Templeton NS, Medina D and Zhang M . (2003). Histol. Histopathol., 18, 201–206.
Shibata MA, Liu ML, Knudson MC, Shibata E, Yoshidome K, Bandey T, Korsmeyer SJ and Green JE . (1999). EMBO J., 18, 2692–2701.
Siegel PM, Shu W, Cardiff RD, Muller WJ and Massague J . (2003). Proc. Natl. Acad. Sci. USA, 100, 8430–8435.
Streuli CH and Gilmore AP . (1999). J. Mammary Gland Biol. Neoplasia, 4, 183–191.
Webster MA and Muller WJ . (1994). Semin. Cancer Biol., 5, 69–76.
Yin C, Knudson CM, Korsmeyer SJ and Van Dyke T . (1997). Nature, 385, 637–640.
Acknowledgements
This work was supported by the Howard Hughes Medical Institute and a Howard Temin K01 career award from the National Cancer Institute (to SS Martin).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, S., Ridgeway, A., Pinkas, J. et al. A cytoskeleton-based functional genetic screen identifies Bcl-xL as an enhancer of metastasis, but not primary tumor growth. Oncogene 23, 4641–4645 (2004). https://doi.org/10.1038/sj.onc.1207595
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207595
- Springer Nature Limited
Keywords
This article is cited by
-
BCL-2 protein family: attractive targets for cancer therapy
Apoptosis (2023)
-
PIM3 kinase promotes tumor metastasis in hepatoblastoma by upregulating cell surface expression of chemokine receptor cxcr4
Clinical & Experimental Metastasis (2022)
-
Distinct roles of tumor associated mutations in collective cell migration
Scientific Reports (2021)
-
The apoptosis inhibitor Bcl-xL controls breast cancer cell migration through mitochondria-dependent reactive oxygen species production
Oncogene (2020)
-
BCL-XL overexpression promotes tumor progression-associated properties
Cell Death & Disease (2017)