Abstract
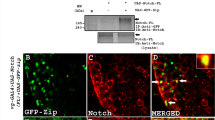
The actin filament-associated protein of 110 kDa (AFAP-110) is a Src binding partner that represents a potential modulator of actin filament integrity in response to cellular signals. Previous reports have demonstrated that AFAP-110 is capable of directly binding and altering actin filaments. Deletion of the leucine zipper motif of AFAP-110 (AFAP-110Δlzip) has been shown to induce a phenotype which resembles Src-transformed cells, by repositioning actin filaments into rosettes. This deletion also mimics a conformational change in AFAP-110 that is detected in Src-transformed cells. The results presented here indicate that unlike AFAP-110, AFAP-110Δlzip is capable of activating cellular tyrosine kinases, including Src family members, and that AFAP-110Δlzip itself is hyperphosphorylated. The newly tyrosine phosphorylated proteins and activated Src-family members appear to be associated with actin-rich lamellipodia. A point mutation that alters the SH3-binding motif of AFAP-110Δlzip prevents it from activating tyrosine kinases and altering actin filament integrity. In addition, a deletion within a pleckstrin homology (PH) domain of AFAP-110Δlzip will also revert its effects upon actin filaments. Lastly, dominant-positive RhoAV14 will block the ability of AFAP-110Δlzip from inducing actin filament rosettes, but does not inhibit Src activation. Thus, conformational changes in AFAP-110 enable it to activate cellular kinases in a mechanism requiring SH3 and/or PH domain interactions. We hypothesize that cellular signals which alter AFAP-110 conformation, enable it to activate cellular kinases such as cSrc, which then direct changes in actin filament integrity in a Rho-dependent fashion.









Similar content being viewed by others
References
Baisden JM, Qian Y, Zot HM, Flynn DC . 2001 Oncogene in press
Bolen JB, Veillette A, Schwartz AM, DeSeau V, Rosen N . 1987 Proc. Natl. Acad. Sci. USA 84: 2251–2255
Boschek CB, Jockusch BM, Friis RR, Back R, Grundmann E, Bauer H . 1981 Cell 24: 175–184
Boyle WJ, van der GP, Hunter T . 1991 Methods Enzymol. 201: 110–149
Briggs SD, Sharkey M, Stevenson M, Smithgall TE . 1997 J. Biol. Chem. 272: 17899–17902
Brown MT, Cooper JA . 1996 Biochim. Biophys. Acta 1287: 121–149
Cartwright CA, Meisler AI, Eckhart W . 1990 Proc. Natl. Acad. Sci. USA 87: 558–562
Felice GR, Eason P, Nermut MV, Kellie S . 1990 Eur. J. Cell Biol. 52: 47–59
Fincham VJ, Chudleigh A, Frame MC . 1999 J Cell Sci. 112: Pt 6 947–956
Flynn DC, Leu TH, Reynolds AB, Parsons JT . 1993 Mol. Cell Biol. 13: 7892–7900
Guappone AC, Flynn DC . 1997 Mol. Cell Biochem. 175: 243–252
Guappone AC, Weimer T, Flynn DC . 1998 Mol. Carcinog. 22: 110–119
Hamaguchi M, Hanafusa H . 1987 Proc. Natl. Acad. Sci. USA 84: 2312–2316
He H, Hirokawa Y, Levitzki A, Maruta H . 2000 Cancer J 6: 243–248
Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ . 1999 Nat. Genet. 21: 187–190
Kanner SB, Reynolds AB, Wang HC, Vines RR, Parsons JT . 1991 EMBO J. 10: 1689–1698
Liu X, Pawson T . 1994 Recent Prog. Horm. Res. 49: 149–160
Mayer T, Meyer M, Janning A, Schiedel AC, Barnekow A . 1999 Oncogene 18: 2117–2128
Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT . 1997 Nature 385: 650–653
Qian Y, Baisden JM, Westin EH, Guappone AC, Koay TC, Flynn DC . 1998 Oncogene 16: 2185–2195
Qian Y, Guappone AC, Baisden JM, Hill MW, Summy J, Flynn DC . 1999 Hybridoma 18: 167–175
Qian Y, Baisden JM, Zot HG, Van Winkle WB, Flynn DC . 2000 Exp. Cell Res. 255: 102–113
Reynolds AB, Kanner SB, Wang HC, Parsons JT . 1989 Mol. Cell Biol. 9: 3951–3958
Ridley AJ, Hall A . 1992 Cell 70: 389–399
Rosen N, Bolen JB, Schwartz AM, Cohen P, DeSeau V, Israel MA . 1986 J. Biol. Chem. 261: 13754–13759
Sabe H, Okada M, Nakagawa H, Hanafusa H . 1992 Mol. Cell Biol. 12: 4706–4713
Schwartzberg PL, Xing L, Hoffmann O, Lowell CA, Garrett L, Boyce BF, Varmus HE . 1997 Genes Dev. 11: 2835–2844
Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC . 1985 Exp. Cell Res. 159: 141–157
Thomas JW, Ellis B, Boerner RJ, Knight WB, White GC, Schaller MD . 1998 J Biol. Chem. 273: 577–583
Xing L, Ge C, Zeltser R, Maskevitch G, Mayer BJ, Alexandropoulos K . 2000 Mol. Cell Biol. 20: 7363–7377
Acknowledgements
Thanks to Ray Mattingly, Wayne State University for the dominant-positive RhoAV14 constructs. This work was supported by a grant from the NCI (to DC Flynn), CA60731. JM Baisden was supported by a grant from the West Virginia University Medical Scientist Training Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baisden, J., Gatesman, A., Cherezova, L. et al. The intrinsic ability of AFAP-110 to alter actin filament integrity is linked with its ability to also activate cellular tyrosine kinases. Oncogene 20, 6607–6616 (2001). https://doi.org/10.1038/sj.onc.1204802
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204802
- Springer Nature Limited
Keywords
This article is cited by
-
lncRNA AFAP1-AS1 promotes triple negative breast cancer cell proliferation and invasion via targeting miR-145 to regulate MTH1 expression
Scientific Reports (2020)
-
Up-regulated lncRNA AFAP1-AS1 indicates a poor prognosis and promotes carcinogenesis of breast cancer
Breast Cancer (2019)
-
RETRACTED ARTICLE: Upregulation of the long non-coding RNA AFAP1-AS1 affects the proliferation, invasion and survival of tongue squamous cell carcinoma via the Wnt/β-catenin signaling pathway
Molecular Cancer (2018)
-
Long non-coding RNA AFAP1-AS1 plays an oncogenic role in promoting cell migration in non-small cell lung cancer
Cellular and Molecular Life Sciences (2018)
-
Long Noncoding RNA AFAP1-AS1 Promotes Cell Proliferation and Apoptosis of Gastric Cancer Cells via PTEN/p-AKT Pathway
Digestive Diseases and Sciences (2017)