Abstract
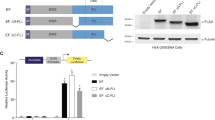
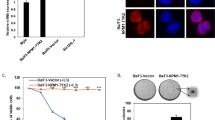
The t(11; 22)(p13; q12) translocation associated with desmosplastic small round cell tumor results in a chimeric molecule fusing the amino terminal domain (NTD) of the EWS1 gene to three of the four carboxy-terminal zinc fingers of the WT1 tumor suppressor gene. Since the DNA binding domains of WT1 and EWS/WT1 are structurally different, we have assessed the functional consequences of the EWS/WT1 fusion. We find that the EWS/WT1 protein has a higher binding affinity for a given recognition target than the WT1 product. This is unlike other fusion products involving translocation of the NTD of EWS to DNA binding domains in which DNA binding specificity and affinity is not changed. We demonstrate that EWS/WT1 is a nuclear protein and that the NTD of EWS contains (a) nuclear localization signal(s). We also find that the integrity of a domain within the WT1 zinc fingers, responsible for mediating interaction between WT1 and the transcriptional repressor par-4, is disrupted in the EWS/WT1 fusion product. Deletion analysis of the NTD of EWS indicated that integrity of the entire domain was necessary to achieve full transactivation potential.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, J., Lee, K. & Pelletier, J. The DNA binding domains of the WT1 tumor suppressor gene product and chimeric EWS/WT1 oncoprotein are functionally distinct. Oncogene 16, 1021–1030 (1998). https://doi.org/10.1038/sj.onc.1201616
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1201616
- Springer Nature Limited
Keywords
This article is cited by
-
Salt-Inducible Kinase 1 is a potential therapeutic target in Desmoplastic Small Round Cell Tumor
Oncogenesis (2022)
-
EWS-Oct-4B, an alternative EWS-Oct-4 fusion gene, is a potent oncogene linked to human epithelial tumours
British Journal of Cancer (2010)
-
PDGF-A, PDGF-Rβ, TGFβ3 and bone morphogenic protein-4 in desmoplastic small round cell tumors with EWS-WT1 gene fusion product and their role in stromal desmoplasia: an immunohistochemical study
Modern Pathology (2005)
-
Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax : Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity
Oncogene (2005)
-
Induction of the interleukin-2/15 receptor β-chain by the EWS–WT1 translocation product
Oncogene (2002)