Abstract
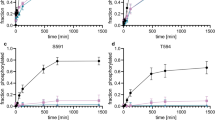
In vivo p53 is multiply phosphorylated by different protein kinases suggesting a central role for phosphorylation in modulating p53 function. In addition, p53 was found to be associated with two protein kinases, p34cdc2 and protein kinase CK2. Here we report the precise mapping of the interaction sites of p53 – p34cdc2 complexes. The p34cdc2 binding site on human p53 maps to one distinct C-terminal site LQIRGRERFE (aa 330 – 339) close to the corresponding phosphorylation site at serine 315. In order to test whether phosphorylation of p53 might influence the binding of p53 to p34cdc2 phosphorylation mutants of the C-terminus of p53, which mimick permanent phosphorylation, were tested on their ability to bind to p34cdc2 in vitro. Substitution of serine 315 (the p34cdc2 phosphorylation site) with aspartic acid had only little effect on complex formation whereas an exchange of serine 392 (the protein kinase CK2 phosphorylation site) to aspartic acid resulted in a significant reduced relative binding affinity of p53 to p34cdc2. The same result was obtained when the C-terminus of p53 was phosphorylated by purified protein kinase CK2 prior to examination of complex formation. In addition, the specificity of the complex formation has been checked by competition experiments with full length p53 proteins and the influence of cyclin B on complex formation was examined.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wagner, P., Fuchs, A., Götz, C. et al. Fine mapping and regulation of the association of p53 with p34cdc2. Oncogene 16, 105–111 (1998). https://doi.org/10.1038/sj.onc.1201510
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1201510
- Springer Nature Limited