Abstract
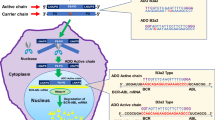
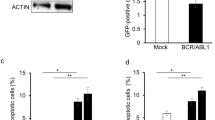
We compared antisense phosphorothioate oligonucleotides (PS-ODN) that target BCL-2 such as Genasense® (G3139-PS), with other PS-ODN or phosphodiester-ODN (PO-ODN) in their relative capacity to induce apoptosis of chronic lymphocytic leukemia (CLL) B cells in vitro. Surprisingly, we found that thymidine-containing PS-ODN, but not PO-ODN, induced activation and apoptosis of CLL cells independent of BCL-2 antisense sequence or CpG motifs. All tested thimidine-containing PS-ODN, irrespective of their primary sequences, reduced the expression of Bcl-2 protein and increased the levels of the proapoptotic molecules p53, Bid, Bax in CLL cells. Apoptosis induced by thymidine-containing PS-ODN was preceded by cellular activation, could be blocked by the tyrosine-kinase inhibitor imatinib mesylate (Gleevec®), and was dependent on ABL kinase. We conclude that thymidine-containing PS-ODN can activate CLL cells and induce apoptosis via a mechanism that is independent of BCL-2 gene interference or CpG motifs.





Similar content being viewed by others
References
Wagner RW . Gene inhibition using antisense oligodeoxynucleotides. Nature 1994; 372: 333–335.
Donis-Keller H . Site specific enzymatic cleavage of RNA. Nucleic Acids Res 1979; 7: 179–192.
Crooke ST . Progress in antisense technology. Annu Rev Med 2004; 55: 61–95.
Stephens AC, Rivers RP . Antisense oligonucleotide therapy in cancer. Curr Opin Mol Ther 2003; 5: 118–122.
Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC . bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood 1993; 82: 1820–1828.
Reed JC . Dysregulation of apoptosis in cancer. J Clin Oncol 1999; 17: 2941–2953.
Reed JC, Stein C, Subasinghe C, Haldar S, Croce CM, Yum S et al. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res 1990; 50: 6565–6570.
Cotter FE, Johnson P, Hall P, Pocock C, al Mahdi N, Cowell JK et al. Antisense oligonucleotides suppress B-cell lymphoma growth in a SCID-hu mouse model. Oncogene 1994; 9: 3049–3055.
Klasa RJ, Gillum AM, Klem RE, Frankel SR . Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev 2002; 12: 193–213.
Rudin CM, Kozloff M, Hoffman PC, Edelman MJ, Karnauskas R, Tomek R et al. Phase I study of G3139, a bcl-2 antisense oligonucleotide, combined with carboplatin and etoposide in patients with small-cell lung cancer. J Clin Oncol 2004; 22: 1110–1117.
Marcucci G, Stock W, Dai G, Klisovic RB, Liu S, Klisovic MI et al. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: pharmacokinetics, pharmacodynamics, and clinical activity. J Clin Oncol 2005; 23: 3404–3411.
Tolcher AW, Chi K, Kuhn J, Gleave M, Patnaik A, Takimoto C et al. A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res 2005; 11: 3854–3861.
Reed JC . Promise and problems of Bcl-2 antisense therapy. J Natl Cancer Inst 1997; 89: 988–990.
Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995; 374: 546–549.
Kato K, Cantwell MJ, Sharma S, Kipps TJ . Gene transfer of CD40-ligand induces autologous immune recognition of chronic lymphocytic leukemia B cells. J Clin Invest 1998; 101: 1133–1141.
Matutes E, Owusu-Ankomah K, Morilla R, Garcia Marco J, Houlihan A, Que TH et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia 1994; 8: 1640–1645.
Raynaud FI, Orr RM, Goddard PM, Lacey HA, Lancashire H, Judson IR et al. Pharmacokinetics of G3139, a phosphorothioate oligodeoxynucleotide antisense to bcl-2, after intravenous administration or continuous subcutaneous infusion to mice. J Pharmacol Exp Ther 1997; 281: 420–427.
Castro JE, Motta M, Wu C, Carson DA, Kipps TJ . Phosphorothioate oligodeoxynucleotides activate chronic lymphocytic leukemia B cells through a CpG independent mechanism. Blood 2002; 100: 1463a.
Wu CC, Castro JE, Motta M, Cottam HB, Kyburz D, Kipps TJ et al. Selection of oligonucleotide aptamers with enhanced uptake and activation of human leukemia B cells. Hum Gene Ther 2003; 14: 849–860.
Krajewski S, Bodrug S, Gascoyne R, Berean K, Krajewska M, Reed JC . Immunohistochemical analysis of Mcl-1 and Bcl-2 proteins in normal and neoplastic lymph nodes. Am J Pathol 1994; 145: 515–525.
Svingen PA, Karp JE, Krajewski S, Mesner Jr PW, Gore SD, Burke PJ et al. Evaluation of Apaf-1, and procaspases-2, -3, -7, -8, and -9 as potential prognostic markers in acute leukemia. Blood 2000; 96: 3922–3931.
Chu P, Deforce D, Pedersen IM, Kim Y, Kitada S, Reed JC et al. Latent sensitivity to Fas-mediated apoptosis after CD40 ligation may explain activity of CD154 gene therapy in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2002; 99: 3854–3859.
Cantwell MJ, Sharma S, Friedmann T, Kipps TJ . Adenovirus vector infection of chronic lymphocytic leukemia B cells. Blood 1996; 88: 4676–4683.
Baumgarth N . A two-phase model of B-cell activation. Immunol Rev 2000; 176: 171–180.
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol 2004; 110: 252–266.
Radloff M, Gercken G . Protein kinase C activity and phosphoprotein pattern in stimulated alveolar macrophages. Toxicol Lett 1996; 88: 139–145.
Vlahos CJ, Matter WF, Hui KY, Brown RF . A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 1994; 269: 5241–5248.
Qatsha KA, Rudolph C, Marme D, Schachtele C, May WS . Go 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level-producing reservoir cells in vitro. Proc Natl Acad Sci USA 1993; 90: 4674–4678.
Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Druker BJ et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res 1996; 56: 100–104.
Bakhtiar R, Lohne J, Ramos L, Khemani L, Hayes M, Tse F . High-throughput quantification of the anti-leukemia drug STI571 (Gleevec) and its main metabolite (CGP 74588) in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2002; 768: 325–340.
Pepper C, Thomas A, Hoy T, Cotter F, Bentley P . Antisense-mediated suppression of Bcl-2 highlights its pivotal role in failed apoptosis in B-cell chronic lymphocytic leukaemia. Br J Haematol 1999; 107: 611–615.
Raffo A, Lai JC, Stein CA, Miller P, Scaringe S, Khvorova A et al. Antisense RNA down-regulation of bcl-2 expression in DU145 prostate cancer cells does not diminish the cytostatic effects of G3139 (Oblimersen). Clin Cancer Res 2004; 10: 3195–3206.
Krieg AM . From A to Z on CpG. Trends Immunol 2002; 23: 64–65.
Vollmer J, Janosch A, Laucht M, Ballas ZK, Schetter C, Krieg AM . Highly immunostimulatory CpG-free oligodeoxynucleotides for activation of human leukocytes. Antisense Nucleic Acid Drug Dev 2002; 12: 165–175.
Reed JC . Apoptosis-targeted therapies for cancer. Cancer Cell 2003; 3: 17–22.
Gibson LF, Fortney J, Magro G, Ericson SG, Lynch JP, Landreth KS . Regulation of BAX and BCL-2 expression in breast cancer cells by chemotherapy. Breast Cancer Res Treat 1999; 55: 107–117.
Husain SS, Szabo IL, Tamawski AS . NSAID inhibition of GI cancer growth: clinical implications and molecular mechanisms of action. Am J Gastroenterol 2002; 97: 542–553.
Wang JY . DNA damage and apoptosis. Cell Death Differ 2001; 8: 1047–1048.
Lain S, Lane D . Improving cancer therapy by non-genotoxic activation of p53. Eur J Cancer 2003; 39: 1053–1060.
Pluquet O, Hainaut P . Genotoxic and non-genotoxic pathways of p53 induction. Cancer Lett 2001; 174: 1–15.
Chau BN, Chen TT, Wan YY, DeGregori J, Wang JY . Tumor necrosis factor alpha-induced apoptosis requires p73 and c-ABL activation downstream of RB degradation. Mol Cell Biol 2004; 24: 4438–4447.
Wang JY . Regulation of cell death by the Abl tyrosine kinase. Oncogene 2000; 19: 5643–5650.
Goldberg Z, Vogt Sionov R, Berger M, Zwang Y, Perets R, Van Etten RA et al. Tyrosine phosphorylation of Mdm2 by c-Abl: implications for p53 regulation. EMBO J 2002; 21: 3715–3727.
Vella V, Zhu J, Frasca F, Li CY, Vigneri P, Vigneri R et al. Exclusion of c-Abl from the nucleus restrains the p73 tumor suppression function. J Biol Chem 2003; 278: 25151–25157.
Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin Jr WG, Levrero M et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 1999; 399: 806–809.
Truong T, Sun G, Doorly M, Wang JY, Schwartz MA . Modulation of DNA damage-induced apoptosis by cell adhesion is independently mediated by p53 and c-Abl. Proc Natl Acad Sci USA 2003; 100: 10281–10286.
Yi X, Yin XM, Dong Z . Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem 2003; 278: 16992–16999.
Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ . BID: a novel BH3 domain-only death agonist. Genes Dev 1996; 10: 2859–2869.
Leu JI, Dumont P, Hafey M, Murphy ME, George DL . Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol 2004; 6: 443–450.
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303: 1010–1014.
Acknowledgements
This work was supported by a National Institutes of Health K08 Grant CA106 605-01 (JEC) and PO1 Grant CA 81534 for the CLL Research Consortium (TJK). The authors acknowledge the helpful technical support provided by Dr Anissa Agadir from Pharmingen, La Jolla, CA and Dr Laura Rassenti from the Chronic lymphocytic leukemia Research Consortium (C.R.C)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, J., Prada, C., Aguillon, R. et al. Thymidine-phosphorothioate oligonucleotides induce activation and apoptosis of CLL cells independently of CpG motifs or BCL-2 gene interference. Leukemia 20, 680–688 (2006). https://doi.org/10.1038/sj.leu.2404144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404144
- Springer Nature Limited
Keywords
This article is cited by
-
Targeting the CXCR4 pathway using a novel anti-CXCR4 IgG1 antibody (PF-06747143) in chronic lymphocytic leukemia
Journal of Hematology & Oncology (2017)
-
TLR9 agonists induced cell death in Burkitt's lymphoma cells is variable and influenced by TLR9 polymorphism
Cell Death & Disease (2012)
-
Diagnosing and exploiting cancer's addiction to blocks in apoptosis
Nature Reviews Cancer (2008)
-
The Akt signaling pathway determines the different proliferative capacity of chronic lymphocytic leukemia B-cells from patients with progressive and stable disease
Leukemia (2007)