Abstract
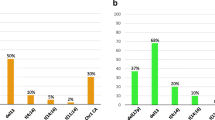
Many patients with t(8;21) AML have residual positive cells during remission. We previously developed D-FISH probes that detect both derivative chromosomes and the normal alleles. In negative controls, only 2/44000 (0.0045%) positive signals were observed. To investigate MRD, we examined specimens from 29 patients who had initially obtained CR. In remission patients, 61% had 1–4/2000 positive cells (0.05–0.19%). Higher frequencies were found in two patients in early relapse and in one patient in early remission. However, a negative test did not exclude relapse. Since false positives were negligible and because most t(8;21) AMLs express CD34, we asked whether cell sorting combined with FISH would increase the sensitivity. In one patient, we observed that 80% of CD34+ cells were t(8;21)+ at 2 months from initial clinical and cytogenetic remission. However, by 5 months the pre- and post-sorted populations contained 0.15% and 0.06% t(8;21) cells, respectively. Whereas essentially all t(8;21) cells in the initial specimen expressed CD34, only 0.6% were subsequently CD34+. These results are consistent with in vitro assays showing that residual t(8;21) cells undergo differentiation. Thus, FISH can identify MRD in a majority of t(8;21) patients and, combined with CD34+ selection, may provide an indirect assessment of the differentiation state of residual t(8;21) cells.


Similar content being viewed by others
References
Walker H, Smith FJ, Betts DR . Cytogenetics in acute myeloid leukaemia Blood Rev 1994 8: 30–36
Nucifora G, Rowley JD . The AML1 and ETO genes in acute myeloid leukemia with a t(8;21) Leuk Lymphoma 1994 14: 353–362
Erickson P, Gao J, Chang KS, Look T, Whisenant E, Raimondi S, Lasher R, Trujillo J, Rowley J, Drabkin H . Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt Blood 1992 80: 1825–1831
Miyoshi H, Kozu T, Shimizu K, Enomoto K, Maseki N, Kaneko Y, Kamada N, Ohki M . The t(8;21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript EMBO J 1993 12: 2715–2721
Chang KS, Fan YH, Stass SA, Estey EH, Wang G, Trujillo JM, Erickson P, Drabkin H . Expression of AML1-ETO fusion transcripts and detection of minimal residual disease in t(8;21)-positive acute myeloid leukemia Oncogene 1993 8: 983–988
Downing JR, Head DR, Curcio-Brint AM, Hulshof MG, Motroni TA, Raimondi SC, Carroll AJ, Drabkin HA, Willman C, Theil KS et al. An AML1/ETO fusion transcript is consistently detected by RNA-based polymerase chain reaction in acute myelogenous leukemia containing the (8;21)(q22;q22) translocation Blood 1993 81: 2860–2865
Nucifora G, Larson RA, Rowley JD . Persistence of the 8;21 translocation in patients with acute myeloid leukemia type M2 in long-term remission Blood 1993 82: 712–715
Miyamoto T, Weissman IL, Akashi K . AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation Proc Natl Acad Sci USA 2000 97: 7521–7526
Tobal K, Liu Yin JA . Molecular monitoring of minimal residual disease in acute myeloblastic leukemia with t(8;21) by RT-PCR Leuk Lymphoma 1998 31: 115–120
Miyamoto T, Nagafuji K, Harada M, Niho Y . Significance of quantitative analysis of AML1/ETO transcripts in peripheral blood stem cells from t(8;21) acute myelogenous leukemia Leuk Lymphoma 1997 25: 69–75
Marcucci G, Livak KJ, Bi W, Strout MP, Bloomfield CD, Caligiuri MA . Detection of minimal residual disease in patients with AML1/ETO-associated acute myeloid leukemia using a novel quantitative reverse transcription polymerase chain reaction assay Leukemia 1998 12: 1482–1489
Tobal K, Newton J, Macheta M, Chang J, Morgenstern G, Evans PA, Morgan G, Lucas GS, Liu Yin JA . Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse Blood 2000 95: 815–819
el-Rifai W, Ruutu T, Vettenranta K, Temtamy S, Knuutila S . Minimal residual disease after allogeneic bone marrow transplantation for chronic myeloid leukaemia: a metaphase-FISH study Br J Haematol 1996 92: 365–369
Varella-Garcia M . Assessing residual leukemia through fluorescence in situ hybridization (FISH) Gen Mol Biol 1998 21: 323–327
Paskulin GA, Philips G, Morgan R, Sandberg A, Richkind K, Borovik C, McGavran L, Rabinovich N, Dietz-Band J, Erickson P, Drabkin H, Varella-Garcia M . Pre-clinical evaluation of probes to detect t(8;21) AML minimal residual disease by fluorescence in situ hybridization Genes Chromosomes Cancer 1998 21: 144–151
Fischer K, Scholl C, Salat J, Frohling S, Schlenk R, Bentz M, Stilgenbauer S, Lichter P, Dohner H . Design and validation of DNA probe sets for a comprehensive interphase cytogenetic analysis of acute myeloid leukemia Blood 1996 88: 3962–3971
Dewald GW, Wyatt WA, Juneau AL, Carlson RO, Zinsmeister AR, Jalal SM, Spurbeck JL, Silver RT . Highly sensitive fluorescence in situ hybridization method to detect double BCR/ABL fusion and monitor response to therapy in chronic myeloid leukemia Blood 1998 91: 3357–3365
Andrieu V, Radford-Weiss I, Troussard X, Chane C, Valensi F, Guesnu M, Haddad E, Viguier F, Dreyfus F, Varet B, Flandrin G, Macintyre E . Molecular detection of t(8;21)/AML1-ETO in AML M1/M2: correlation with cytogenetics, morphology and immunophenotype Br J Haematol 1996 92: 855–865
Kita K, Shirakawa S, Kamada N . Cellular characteristics of acute myeloblastic leukemia associated with t(8;21)(q22;q22). The Japanese Cooperative Group of Leukemia/Lymphoma Leuk Lymphoma 1994 13: 229–234
Tobal K, Liu Yin JA . RT-PCR method with increased sensitivity shows persistence of PML-RARA fusion transcripts in patients in long-term remission of APL Leukemia 1998 12: 1349–1354
Shimizu K, Kitabayashi I, Kamada N, Abe T, Maseki N, Suzukawa K, Ohki M . AML1-MTG8 leukemic protein induces the expression of granulocyte colony-stimulating factor (G-CSF) receptor through the up-regulation of CCAAT/enhancer binding protein epsilon Blood 2000 96: 288–296
Rhoades KL, Hetherington CJ, Harakawa N, Yergeau DA, Zhou L, Liu LQ, Little MT, Tenen DG, Zhang DE . Analysis of the role of AML1-ETO in leukemogenesis, using an inducible transgenic mouse model Blood 2000 96: 2108–2015
Cai Z, de Bruijn M, Ma X, Dortland B, Luteijn T, Downing JR, Dzierzak E . Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo Immunity 2000 13: 423–431
Bonnet D, Dick JE . Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell Nat Med 1997 3: 730–737
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE . A cell initiating human acute myeloid leukaemia after transplantation into SCID mice Nature 1994 367: 645–648
Acknowledgements
We wish to acknowledge the assistance of the Flow Cytometry and Cytogenetics Cores of the University of Colorado Cancer Center. M Andreeff was supported by NIH PO1 CA 55164.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Varella-Garcia, M., Hogan, C., Odom, L. et al. Minimal residual disease (MRD) in remission t(8;21) AML and in vivo differentiation detected by FISH and CD34+ cell sorting. Leukemia 15, 1408–1414 (2001). https://doi.org/10.1038/sj.leu.2402219
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402219
- Springer Nature Limited