Abstract
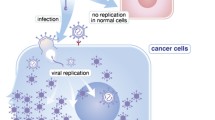
The presence of severe hypoxia and necrosis in solid tumors offers the potential to apply an anaerobic bacterial enzyme/prodrug approach in cancer treatment. In this context the apathogenic C. acetobutylicum was genetically engineered to express and secrete E. coli cytosine deaminase (CDase). Considerable levels of functional cytosine deaminase were detected in lysates and supernatants of recombinant C acetobutylicum cultures. After administration of the recombinant Clostridium to rhabdomyosarcoma bearing rats used as a model, cytosine deaminase could be detected at the tumor site. Moreover, following administration of the vascular targeting agent combretastatin A-4 phosphate significantly increased levels of cytosine deaminase were detected at the tumor site as a consequence of enlarged tumor necrosis and subsequently improved growth of C. acetobutylicum. The results provide evidence for the potential application of Clostrisdium-based therapeutic protein transfer to tumors in anticancer therapy. Cancer Gene Therapy (2001) 8, 294–297
Similar content being viewed by others
References
Brown JM, Giaccia AJ . The unique physiology of solid tumors: opportunities and problems for cancer therapy Cancer Res 1998; 58:: 1408–1416
Theys J, Nuyts S, Landuyt W, et al . Stable Escherichia coli–Clostridium acetobutylicum shuttle vector for secretion of murine tumor necrosis factor alpha Appl Environ Microbiol 1999; 65:: 4295–4300
Haack K, Moebius U, Knebel Doeberitz MV, et al . Detection of cytosine deaminase in genetically modified tumor cells by specific antibodies Hum Gene Ther 1997; 8:: 1395–1401
Landuyt W, Verdoes O, Darius DO, et al . Vascular targeting of solid tumors: a major “inverse” volume–response relationship following combretastatin A-4 phosphate treatment of rat rhabdomyosarcomas Eur J Cancer 2000; 36:: 1833–1843
Lambin P, Nuyts S, Landuyt W, et al . The potential therapeutic gain of radiation-associated gene therapy with the suicide gene cytosine deaminase Int J Radiat Biol 2000; 76:: 285–293
Acknowledgements
G. Reysset (Institut Pasteur, Paris) is acknowledged for his help on the transformation of C. acetobutylicum NI 4082. We also appreciate the stimulating discussions with Ernst de Bruijn from the Oncology Department (University Hospital Gasthuisberg, Leuven). We acknowledge the financial support from “Het Fonds voor Wetenschappelijk Onderzoek-Vlaanderen,” “Het K.U. Leuven Onderzoeksfonds,” and “VIS (Verkennende Internationale Samenwerking).” Jan Theys and Sandra Nuyts are research fellows of “IWT” (Vlaams Instituut voor de bevordering van het Wetenschappelijk-Technologisch Onderzoek in de Industrie).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theys, J., Landuyt, W., Nuyts, S. et al. Specific targeting of cytosine deaminase to solid tumors by engineered Clostridium acetobutylicum. Cancer Gene Ther 8, 294–297 (2001). https://doi.org/10.1038/sj.cgt.7700303
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700303
- Springer Nature America, Inc.
Keywords
This article is cited by
-
Multiplex genetic manipulations in Clostridium butyricum and Clostridium sporogenes to secrete recombinant antigen proteins for oral-spore vaccination
Microbial Cell Factories (2024)
-
Bacterial therapies at the interface of synthetic biology and nanomedicine
Nature Reviews Bioengineering (2023)
-
Current advances in microbial-based cancer therapies
Medical Oncology (2023)
-
Engineering the gut microbiota to treat chronic diseases
Applied Microbiology and Biotechnology (2020)
-
Intestinal probiotics E. coli Nissle 1917 as a targeted vehicle for delivery of p53 and Tum-5 to solid tumors for cancer therapy
Journal of Biological Engineering (2019)