Summary:
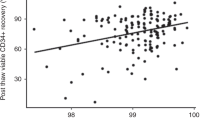
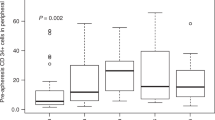
In all, 78 peripheral hematopoietic progenitor cell collections from 52 patients were evaluated using our previously published validated post-thaw assays at the time of collection and following transplantation by assessment of viable CD34+ cells, and granulocyte–macrophage colony-forming units (CFU-GM) cryopreserved in quality control vials. The median (range) post-thaw recovery of viable CD34+ cells and CFU-GM was 66.4% (36.1–93.6%) and 63.0% (28.6–85.7%), respectively, which did not show significant correlation with the engraftment of either neutrophils (P=0.136 and 0.417, respectively) or platelets (P=0.88 and 0.126, respectively). However, the reinfused viable CD34+ cells/kg of patient weight pre- or post-cryopreservation showed significant correlation to engraftment of neutrophils (P=0.0001 and 0.001, respectively) and platelets (P=0.023 and 0.010, respectively), whereas CFU-GM pre- or post-cryopreservation was significantly correlated to neutrophils (P=0.011 and 0.007, respectively) but not to platelets (P=0.112 and 0.100, respectively). The results show that post-cryopreservation assessment of viable CD34+ cells or CFU-GM is as reliable a predictor of rapid engraftment as that of pre-cryopreservation measures. Therefore, the post-cryopreservation number of viable CD34+ cells or CFU-GM should be used to eliminate the risks of unforeseen cell loss that could occur during cryopreservation or long-term storage.





Similar content being viewed by others
References
Hass R, Mohle R, Fruhauf S et al. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma. Blood 1994; 83: 3787–3794.
To LB, Haylock DN, Simmons PJ et al. The biology and clinical uses of blood stem cells. Blood 1997; 89: 2233–2258.
Bender JG, To LB, Williams S et al. Defining a therapeutic dose of peripheral blood stem cells. J Hematother 1992; 1: 329–341.
Humpe A, Riggert J, Vehmeyer K et al. Comparison of CD34+ cell number and colony growth before and after cryopreservation of peripheral blood progenitor and stem cell harvests: influence of prior chemotherapy. Transfusion 1997; 37: 1050–1057.
Fietz T, Reufi B, Mucke C et al. Flow cytometric CD34+ determination in stem cell transplantation: before or after cryopreservation of grafts? J Hematother Stem Cell Res 2002; 11: 429–435.
Feugier P, Bensoussan D, Girard F et al. Hematologic recovery after autologous PBSC transplantation: importance of the number of post-thaw CD34+ cells. Transfusion 2003; 43: 878–884.
Allan DS, Keeney M, Howson-Jan K et al. Number of viable CD34+ cells infused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 29: 967–972.
Beanjean F, Bourhis JH, Bayle CH et al. Successful cryopreservation of purified autologous CD34+ cells: influence of freezing parameters on cell recovery and engraftment. Bone Marrow Transplant 1998; 22: 1091–1096.
Yang H, Acker JP, Hannon J et al. Damage and protection of UC blood cells during cryopreservation. Cytherapy 2001; 3: 377–386.
Yang H, Acker JP, Cabuhat M et al. Effects of incubation temperature and time after thawing on viability assessment of peripheral hematopoietic progenitor cells cryopreserved for transplantation. Bone Marrow Transplant 2003; 32: 1021–1026.
Crowley JP, Rene A, Valeri CR . The recovery, structure, and function of human blood leukocytes after freeze-preservation. Cryobiology 1974; 11: 395–409.
Armitage WJ, Mazur P . Toxic and osmotic effects of glycerol and human granulocytes. Am J Physiol 1984; 247 (Cell Physiol. 16): C382–C389.
Yang H, Zhao H, McGann LE . Effects of DMSO on flow cytometric absolute CD34+ cell enumeration. Cytotherapy 2003; 5: 453.
Keeney M, Chin-Yee I, Weir K et al. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guideline. Cytometry 1998; 34: 61–70.
Lefree F, Makki J, Marolleau HP et al. CD34+ cells during leukapheresis procedures: relationship of volume processed and quantity of peripheral blood progenitor cells collected. Transfusion 2000; 40: 493–494.
To LB, Haylock DN, Simmons PJ et al. The biology and clinical uses of blood stem cells. Blood 1997; 89: 2233–2258.
Bender JG, To LB, Williams S et al. Defining a therapeutic dose of peripheral blood stem cells. J Hematother 1992; 1: 329–341.
Montanari M, Capelli D, Poloni A et al. Long-term hematologic reconstitution after autologous peripheral blood progenitor cell transplantation: a comparison between controlled-rate freezing and uncontrolled-rate freezing at −80°C. Transfusion 2003; 43: 42–49.
Bender JG, Lum L, Unverzagt KL et al. Correlation of colony-forming cells, long term culture initiating cells and CD34+ cells in apheresis products from patients mobilized for peripheral blood progenitors with different regimens. Bone Marrow Transplant 1994; 13: 479–485.
Hubel A, Carlquist D, Clay M, McCullough J . Liquid storage, shipment, and cryopreservation of cord blood. Transfusion 2004; 44: 518–525.
Feugier P, Bensoussan D, Girard F et al. Hematologic recovery after autologous PBPC transplantation: importance of the number of postthaw CD34+cells. Transfusion 2003; 43: 878–884.
Broxmeyer HE, Hangoc G, Cooper S et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci USA 1992; 89: 4109–4113.
Broxmeyer HE, Cooper S . High-efficiency recovery of immature haematopoietic progenitor cells with extensive proliferative capacity from human cord blood cryopreserved for 10 years. Clin Exp Immunol 1997; 107 (Suppl. 1): 45–53.
Broxmeyer HE, Srour EF, Hangoc G et al. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci USA 2003; 100: 645–650.
Acknowledgements
This work was supported by Grant XE00013 from the Canadian Blood Services Research & Development program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Acker, J., Cabuhat, M. et al. Association of post-thaw viable CD34+ cells and CFU-GM with time to hematopoietic engraftment. Bone Marrow Transplant 35, 881–887 (2005). https://doi.org/10.1038/sj.bmt.1704926
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704926
- Springer Nature Limited
Keywords
This article is cited by
-
Post thawing viable CD34+ Cells dose is a better predictor of clinical outcome in lymphoma patients undergoing autologous stem cell transplantation
Bone Marrow Transplantation (2022)
-
Leukocytapheresis variables and transit time for allogeneic cryopreserved hpc: better safe than sorry
Bone Marrow Transplantation (2022)
-
Allogeneic transplant procurement in the times of COVID-19: Quality report from the central European cryopreservation site
Journal of Translational Medicine (2021)
-
Microfluidic Sorting of Cells by Viability Based on Differences in Cell Stiffness
Scientific Reports (2017)
-
miR-199b, a novel tumor suppressor miRNA in acute myeloid leukemia with prognostic implications
Experimental Hematology & Oncology (2015)