Abstract
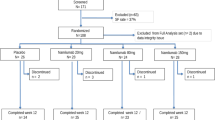
Autologous haemopoietic stem cell transplantation (HSCT) represents a potential therapy for severe rheumatoid arthritis (RA). As a prelude to clinical trails, the safety and efficacy of haemopoietic stem cell (HSC) mobilisation required investigation as colony-stimulating factors (CSFs) have been reported to flare RA. A double-blind, randomised placebo-controlled dose escalation study was performed. Two cohorts of eight patients fulfilling strict eligibility criteria for severe active RA (age median 40 years, range 24–60 years; median disease duration 10.5 years, range 2–18 years) received filgrastim (r-Hu-methionyl granulocyte(G)-CSF) at 5 and 10 μg/kg/day, randomised in a 5:3 ratio with placebo. Patients were unblinded on the fifth day of treatment and those randomised to filgrastim underwent cell harvesting (leukapheresis) daily until 2 × 106/kg CD34+ cells (haemopoietic stem and progenitor cells) were obtained. Patients were assessed by clinical and laboratory parameters before, during and after filgrastim administration. RA flare was defined as an increase of 30% or more in two of the following parameters: tender joint count, swollen joint count or pain score. Efficacy was assessed by quantitation of CD34+ cells and CFU-GM. One patient in the 5 μg/kg/day group and two patients in the 10 μg/kg/day group fulfilled criteria for RA flare, although this did not preclude successful stem cell collection. Median changes in swollen and tender joint counts were not supportive of filgrastim consistently causing exacerbation of disease, but administration of filgrastim at 10 μg/kg/day was associated with rises in median C-reactive protein and median rheumatoid factor compared with placebo. Other adverse events were well recognised for filgrastim and included bone pain (80%) and increases in alkaline phosphatase (four-fold) and lactate dehydrogenase (two-fold). With respect to efficacy, filgrastim at 10 μg/kg/day was more efficient with all patients (n = 5) achieving target CD34+ cell counts with a single leukapheresis (median = 2.8, range = 2.3–4.8 × 106/kg, median CFU-GM = 22.1, range = 4.2–102.9 × 104/kg), whereas 1–3 leukaphereses were necessary to achieve the target yield using 5 μg/kg/day. We conclude that filgrastim may be administered to patients with severe active RA for effective stem cell mobilisation. Flare of RA occurs in a minority of patients and is more likely with 10 than 5 μg/kg/day. However, on balance, 10 μg/kg/day remains the dose of choice in view of more efficient CD34+ cell mobilisation.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Snowden, J., Biggs, J., Milliken, S. et al. A randomised, blinded, placebo-controlled, dose escalation study of the tolerability and efficacy of filgrastim for haemopoietic stem cell mobilisation in patients with severe active rheumatoid arthritis. Bone Marrow Transplant 22, 1035–1041 (1998). https://doi.org/10.1038/sj.bmt.1701486
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1701486
- Springer Nature Limited
Keywords
This article is cited by
-
Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation
Bone Marrow Transplantation (2012)
-
Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases
Pediatric Research (2012)
-
Hematopoietic SCT for the treatment of multiple sclerosis
Bone Marrow Transplantation (2010)
-
Mobilization, harvesting and selection of peripheral blood stem cells in patients with autoimmune diseases undergoing autologous hematopoietic stem cell transplantation
Bone Marrow Transplantation (2007)
-
Granulocyte colony-stimulating factor and neutrophils—forgotten mediators of inflammatory disease
Nature Clinical Practice Rheumatology (2006)