Abstract
Long COVID has been linked to a decline in physical activity and functional capacity. However, it remains unclear which physical symptoms are associated with specific aspects of movement behaviors and functional capacity. We aimed to investigate the associations of fatigue, dyspnea, post-exertional malaise, myalgia, and the co-occurrence of symptoms with movement behaviors and functional capacity in individuals with Long COVID. A cross-sectional multicenter study was conducted. Questionnaires were used to assess fatigue, dyspnea, post-exertional malaise, and myalgia. Accelerometry was employed to assess sedentary time, steps per day, light physical activity, and moderate-to-vigorous physical activity. The six-minute walk test, 30-s chair stand test, and timed up and go were used to assess functional capacity. One hundred and two community-dwelling individuals who had been living with Long COVID for 15 ± 10 months participated in the study. Fatigue, post-exertional malaise, and the co-occurrence of physical symptoms showed a negative association with step count, while post-exertional malaise was also negatively associated with moderate-to-vigorous physical activity. Dyspnea showed a negative association with the functional score, including all tests. Our findings suggest that fatigue, post-exertional malaise, and the co-occurrence of physical symptoms are negatively associated with physical activity, while dyspnea is negatively associated with functional capacity in individuals with Long COVID.
Similar content being viewed by others
Introduction
Long COVID is characterized by the persistence of symptoms for more than 4 weeks from the onset of the acute phase of COVID-191, which can persist for more than 2 years2. Approximately 50% of individuals hospitalized with COVID-19 develop Long COVID3. Additionally, Long COVID is commonly reported by individuals who were not hospitalized during the acute phase of COVID-194. Thus, Long COVID became a global public health issue due to its high prevalence and association with several adverse health-related outcomes, such as rehospitalization and mortality5,6,7. Individuals with Long COVID may experience several concurrent adverse physical symptoms, including fatigue, dyspnea, post-exertional malaise, and myalgia3, making the rehabilitation process extremally challenging.
Individuals experiencing adverse physical symptoms of Long COVID may exhibit intolerance to physical effort8,9,10, contributing to their reduced physical activity and functional capacity. Previous studies have indicated that physical symptoms of Long COVID are linked with reduced physical activity11 and components of functional capacity, such as cardiorrespiratory fitness12,13 and muscle strength13,14. However, these studies relied on self-reported methods to assess physical activity but did not consider other movement behavior components, such as sedentary time or step count and focused on specific components of functional capacity. The accelerometry method provides a comprehensive assessment of movement behaviors, including both physical activity and sedentary time15,16. In the same context, functional capacity may be better assessed by including various components, such as proxies for cardiorespiratory fitness, muscle strength, and dynamic balance. Therefore, this study aimed to investigate the association of adverse physical symptoms (i.e., fatigue, dyspnea, post-exertional malaise, and myalgia) with movement behaviors and various components of functional capacity in individuals with Long COVID. This exploratory study may provide indications about individuals predisposed to low physical activity, high sedentary time, and poor functional capacity among those with Long COVID. Thus, our hypothesis is that the presence of adverse physical symptoms would be associated with lower levels of physical activity, higher levels of sedentary time, and poorer functional capacity.
Material and methods
Study design
This is an exploratory cross-sectional multicenter study, which utilized baseline data from two clinical trials investigating the effects of supervised (Brazilian Registry of Clinical Trials: RBR-10y6jhrs) and semi-supervised (Brazilian Registry of Clinical Trials: RBR-45cbv3) multicomponent exercise training programs on adverse physical symptoms and functional capacity in individuals with Long COVID. Both studies received approval from the Institutional Ethics. The supervised trial was approved by ethics committee of the Federal University of Santa Catarina (CAAE 49487721.9.0000.0121). The semi-supervised trial was approved by ethics committee of the Federal University of Rio Grande do Norte (CAAE 54091521.4.1001.5537). Both protocols were in accordance with the Declaration of Helsinki. All patients signed an informed consent form to declare that they are aware and accept to participate in the study. The baseline data used for this study were collected between November 2021 and October 2023. At the beginning of the volunteer recruitment and throughout the study, there were no public policies regarding movement restrictions in Brazil.
Participants
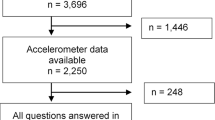
This study included both females and males aged 18 to 75 years who were not engaged in regular physical exercise using the following question: “Have you regularly practiced any physical exercise at least twice per week in the last 3 months? This includes activities such as walking, cycling, gymnastics, sports, etc.”. All participants exhibited at least one of the following adverse physical symptoms for more than 4 weeks after acute COVID-19: fatigue, dyspnea, post-exertional malaise, and myalgia. In the current study, we used the National Institute for Health and Care Excellence (NICE) guidelines to define Long COVID1. According to these guidelines, the term Long COVID refers to signs and symptoms that persist or develop after the acute phase of COVID-19. It encompasses both ongoing symptomatic COVID-19 (lasting from 4 to 12 weeks) and post-COVID-19 syndrome (lasting 12 weeks or more). All patients were diagnosed with COVID-19 based on RT-PCR and serology tests. Both individuals who were hospitalized and those not hospitalized during the acute phase of COVID-19 were considered for inclusion. However, individuals with severe functional limitation (grade 4 on the post-COVID functional scale)17 and dyspnea (score 5 on the modified Medical Research Council scale)18 were excluded from the study. In both centers, participants were recruited for a clinical trial using various methods, including lists of patients treated at the University Hospitals and Primary Health Care Units, as well as media channels such as websites, social media, TV, and radio. The data presented in this study refer to baseline assessments.
Procedures
Information on socioeconomic characteristics, previous chronic conditions, medication use, acute COVID-19, vaccination status, and adverse symptoms of Long COVID (i.e., fatigue, dyspnea, post-exertional malaise, myalgia, insomnia, memory problems, cough, anosmia/ageusia, mental health issues and hair loss) was collected through face-to-face interviews with the participants. For vaccination status, patients who had received at least one booster shot were considered fully vaccinated, while those who had received at least one dose but no booster shots were classified as partially vaccinated. For individuals who were hospitalized during the acute phase of COVID-19, details about the hospitalization, including severity length of hospital stay, oxygen therapy, Intensive Care Unit (ICU) admission, and mechanical ventilation were directly obtained from their medical records.
Exposure
Physical symptoms assessment
Fatigue was assessed using the Chalder Fatigue Scale (cutoff point: score ≥ 4) due to its widespread use and validation in measuring fatigue severity in both clinical and research settings19. Dyspnea was evaluated using the modified Medical Research Council scale, which is a validated tool specifically designed to measure the degree of breathlessness experienced during daily activities18. This scale is commonly used in respiratory research and clinical practice, making it an appropriate choice for assessing dyspnea in Long COVID18. The assessment of post-exertional malaise utilized the DePaul Symptom Questionnaire, a tool developed to measure this specific symptom in patients with myalgia encephalomyelitis/chronic fatigue syndrome (ME/CFS)20. Given the similarity in symptomatology between ME/CFS and Long COVID, the DePaul Symptom Questionnaire was used for assessing post-exertional malaise in our participants. The presence of myalgia was evaluated using the following question: “Are you experiencing any muscle pain that did not exist before the acute COVID-19? The presence of myalgia as an adverse symptom of Long COVID was made with the confirmation that there was no other plausible cause for the muscle pain. As self-reported assessment of myalgia in patients with Long COVID has been the most commonly used method to identify muscle pain in this population21. The co-occurrence of Long COVID physical symptoms (i.e., ≤ 1, 2, 3, and 4 symptoms) was also investigated as an exposure. This approach allows the examination of the cumulative impact of multiple symptoms on the patients’ health, providing a comprehensive understanding of the symptom burden in Long COVID.
Outcome
Accelerometer-measured movement behaviors
Movement behaviors were measured using tri-axial accelerometry (Actigraph GT3X, Actigraph LLC, Pensacola, USA and ActiLife version 6.13.3.2). Participants wore the accelerometer on their right hip for seven consecutive days, removing it during sleeping periods, showering, and water-based activities. They used a logbook to record moments when they removed the accelerometer, went to sleep, and woke up. Non-wear periods were excluded from the analysis, with non-wear time defined as ≥ 90 min of consecutive zero counts and a tolerance of up to 2 min of ≥ 100 counts/min22. For inclusion in the final analysis, participants needed to have at least three valid weekdays of accelerometer wear time and at least one weekend day, with each day requiring ≥ 10 h of wear time. Data were collected at 90 Hz and integrated into 60-s epochs. The cut-offs to define sedentary time, light physical activity, and moderate-to-vigorous physical activity (MVPA) were: 0–99 counts/min, 100–1951 count/min and ≥ 1952 counts/min, respectively16. Although there is no consensus on the cut-off points for assessing movement behaviors in adults aged 18 to 75, we used the aforementioned cut-off points because they are the most commonly employed in studies with adults in the Brazilian population and are also recommended in the guidelines for accelerometer use in Brazil23. Together, these aspects enhance data comparability. The total period (weighted average; weekdays and weekends) was considered to calculate the volumes of sedentary time and physical activity. Step count was calculated as follows: ∑ steps/day ÷ number of valid accelerometer days. The average value of steps per day obtained during the 1-week period was considered for data analysis.
Functional capacity
The functional capacity was assessed using the following tests: (i) six-minute walk test (6MWT); (ii) 30-s chair stand test (30-s CST); (iii) timed up and go (TUG). The 6MWT was conducted in accordance with the recommendations of the American Thoracic Society24. Participants performed two 6MWT with a 30-min interval between them, and the best performance was considered for data analysis. For safety reasons, rating of perceived exertion (Borg scale 6–20), heart rate (Polar H10), blood pressure (OMRON, HEM-7113), and oxygen saturation (oximeter Multilaser HC261) were recorded before, during (at 2 min), and immediately after both 6MWT. Relative values were obtained from an reference equation adjusted for sex, age and body mass index (BMI)25. The CST and TUG were conducted following the recommendations of Rikli and Jones26 and the relative values were obtained adjusted for age and BMI, and age, BMI and height, respectively27. The functional score was represented by a composite score based on the 6MWT, CST, and TUG28. The results of the three tests were standardized into z-scores and summed to create the functional score. The TUG z-scores were multiplied by -1, as the result is presented in seconds and is inversely associated with performance. Individuals with resting blood pressure 160/105 mmHg and/or oxygen saturation < 94% were not allowed to perform the tests for safety reasons.
Data analysis
The data distribution was assessed using the Kolmogorov–Smirnov test. Continuous variables were presented as mean ± standard deviation, while categorical variables were presented as absolute and relative frequencies (%). The independent t-test was utilized to compare movement behaviors and functional capacity between participants with and without each adverse physical symptom. To analyze the association between adverse physical symptoms and accelerometer-measured movement behaviors and functional capacity, a generalized linear model was utilized, with the group without adverse physical symptoms serving as the reference. Collinearity was examined through the Variance Inflation Factor (VIF). The analyses were adjusted for sex, age, income, multimorbidity (i.e., obesity, hypertension, diabetes, hyperlipidemia, cancer, among others)29, ICU admission, and the study center. Obesity was defined as BMI ≥ 30 kg/m2. Additionally, for accelerometer-measured data, adjustments were made for accelerometer wear time. Smoking was not included as a covariate due to the low number of smokers in our sample. The significance level was set at p < 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 27.0.
Results
A total of 102 participants were included in this study (47 ± 13 years; 58.8% females; 29.1 ± 6.0 kg/m2; smokers/ex-smokers: 21.6%). Approximately half of them had post-secondary education (49%) and were receiving 1–3 minimum wages (51%). The majority were employed or studying (74.5%), while 11.8% were retired. The prevalence of hypertension, diabetes, and multimorbidity (≥ 2 chronic conditions) was 28.4%, 15.7%, and 21.6%, respectively. Half of the participants had mild cases (not hospitalized), 19.6% had moderate cases (requiring hospital oxygen therapy), and 30.4% had severe cases (requiring ICU admission) of acute COVID-19. Those who were hospitalized had an average length of stay of 22 ± 18 days, including 16 ± 14 days in the ICU and 13 ± 11.0 days on mechanical ventilation. On average, the participants had been experiencing adverse physical symptoms of Long COVID for 15 ± 10 months (minimum and maximum—1 to 41 month). The most prevalent adverse physical symptoms were fatigue (87.1%), dyspnea (81.2%), post-exertional malaise (58.5%), and myalgia (49.5%). Additionally, the participants reported insomnia (35.5%), memory problems (29.4%), cough (12.7%), anosmia/ageusia (10.8%), mental health issues (anxiety/depression; 7.8%), and hair loss (4.9%). Regarding vaccination status, 67.0% were fully vaccinated (Table 1).
Thirteen patients did not reach the minimum number of days of accelerometer. Two patients reported knee pain and refused to perform the 30-s CST. One participant was unable to complete 6MWT test due to dyspnea and five did not attend the assessment. Movement behaviors revealed that participants spent 10.2 ± 2.0 h/day in sedentary time, 4.8 ± 1.5 h/day in light physical activity, and 0.3 ± 0.2 h/day (113.7 ± 87.2 min/week) in MVPA; 29.5% (n = 26) met the current physical activity guidelines, i.e., at least 150 min/week of MVPA. Additionally, their average step count was 7496 ± 4309 steps/day. Regarding functional capacity, participants covered 518 ± 83 m on the 6MWT, with 72.4% classified as below the estimated value for their age, sex, and BMI. They also performed 13.3 ± 3.1 repetitions on the 30-s CST, with 86% classified as below the estimated value for their age and BMI. Additionally, they completed the TUG in 6.6 ± 1.0 s, with 100% classified as above the estimated value for their age, BMI, and height.
Table 2 details movement behaviors and functional capacity in individuals with Long COVID, categorized according to the presence of different adverse symptoms. Those with fatigue (no = 12,050 ± 5636 steps/day, n = 12; yes = 6707 ± 3570 steps/day, n = 77), post-exertional malaise (no = 9271 ± 5630 steps/day, n = 34; yes = 5993 ± 2500 steps/day, n = 49) and co-occurrence of physical symptoms (≤ 1 symptoms = 12,290 ± 5872, n = 11; 2 symptoms = 7959 ± 4236, n 22; 3 symptoms = 6109 ± 3331, n = 36; 4 symptoms = 6464 ± 2665, n = 19) exhibited a lower number of steps per day compared to their counterparts without these adverse physical symptoms (p < 0.05). Furthermore, individuals experiencing dyspnea performed worse on the TUG (no = 6.1 ± 0.8 s, n = 14; yes = 6.7 ± 1.0 s, n = 80) and had a lower functional score (no = 1.1 ± 1.5 a.u., n = 14; yes = 0.1 ± 1.7 a.u., n = 80) compared to those without this specific symptom (p < 0.05). Additionally, Supplementary Table 1 presents the movement behavior and functional capacity according to the severity of acute COVID-19. Interestingly, individuals with severe cases of acute COVID-19 exhibited a higher number of steps per day compared with mild cases and moderate cases (p < 0.05). There were no differences in functional capacity or other movement behavior variables between the severities of COVID-19.
Table 3 displays the adjusted associations of adverse physical symptoms and movement behaviors in individuals with Long COVID. Fatigue (β = − 3827 steps/day; 95% CI = − 6280 to − 1375), post-exertional malaise (β = − 2864 steps/day; 95% CI = − 4434 to − 1294) and co-occurrence (2 symptoms: β = − 3689 steps/day; − 6329 to − 1049; 3 symptoms: β = − 4754 steps/day; 95% CI = − 7363 to − 2145; 4 symptoms: β = − 5076 steps/day; 95% CI = − 7807 to − 2345) showed a negative association with the number of steps per day (p < 0.05), while post-exertional malaise (β = − 41.4 min/week; 95% CI = − 78.2 to − 4.6) was also negatively associated with MVPA (p < 0.05). No association was found between dyspnea and myalgia with sedentary time, light PA, MVPA or steps count (p > 0.05).
Table 4 displays the adjusted associations of adverse physical symptoms and functional capacity in individuals with Long COVID. Only dyspnea (β = − 0.1 a.u.; 95% CI = − 1.0 to − 0.6) showed a negative association with the functional score (p < 0.05) and (β = 0.5 s; 95% CI = < 0,0 to 1.0) a positive association with TUG. Finally, no association was found between fatigue, post-exertional malaise and myalgia with 6MWT, 30-s CST and TUG (p > 0.05).
Discussion
The main findings of this study were: (i) fatigue and post-exertional malaise were negatively associated with step count; (ii) post-exertional malaise was negatively associated with MVPA; (iii) dyspnea was negatively associated with a total functional score, but none of the adverse physical symptoms were associated with specific functional tests (i.e., 6MWT, 30-s CST, and TUG).
The number of steps per day was ~ 4000 lower in individuals experiencing fatigue. A cohort study revealed that individuals with Long COVID experiencing fatigue had a higher likelihood of being physically inactive 6–11 months following hospitalization30. In other clinical populations, fatigue has also been associated with reduced accelerometer-measured physical activity31,32, reinforcing its negative impact on this aspect of movement behavior. Considering that 87% of our participants were experiencing fatigue, and previous studies have demonstrated that this adverse physical symptom is highly prevalent33,34, our data suggest that individuals with Long COVID are predisposed to the deleterious health consequences of physical inactivity. In our study, 79.5% of the participants did not meet the current physical activity guidelines.
Furthermore, individuals who reported post-exercise malaise demonstrated ~ 3000 fewer steps per day and ~ 41 fewer minutes per week in MVPA. Post-exertional malaise is characterized by a worsening of symptoms that occurs after physical or mental activities, including fatigue, pain, and/or cognitive function20. In our study, 50% of the participants reported experiencing post-exercise malaise. Despite the limitations of a cross-sectional design in establishing causality, the observation that light physical activity levels were comparable between participants with and without this symptom implies that individuals can reduce their MVPA as a protective action against the onset of post-exercise malaise. Our findings are consistent with previous studies8,9 that have demonstrated an association between post-exertional malaise and lower self-reported physical activity levels in individuals with Long COVID.
No significant association was observed between fatigue, post-exertional malaise, and sedentary time, light physical activity, and physical function. All patients were able to perform exercise tests, except for one case that was unable to complete the 6MWT and two patients reported knee pain and refused to perform the 30-s CST. These data suggest that symptomatology does not influence the performance of various exercise tests, consistent with previous literature35,36. However, this finding highlights the potential lack of sensitivity of functional tests in identifying the consequences of Long COVID symptoms on daily activities, as these effects were only evident when measured through physical activity monitors.
Regarding functional capacity, we did not observe significant associations of most adverse physical symptoms with 6MWT, 30-s CST, and TUG. However, individuals experiencing dyspnea demonstrated a lower functional score, which is a composite metric aggregating the performances of all tests. In our study, almost all participants were assessed after 6 months of acute COVID-19. Thus, our findings suggest that the persistence of dyspnea may have a negative impact on the functional capacity in individuals with Long COVID who have been living with this condition for at least 6 months. The functional score included tests that are proxies of cardiorespiratory fitness, lower-limb muscle strength, and agility/dynamic balance26,37, providing a comprehensive view of the relationship between adverse physical symptoms and functional capacity in individuals with Long COVID.
Study limitations
This exploratory study has limitations. The cross-sectional design precludes the establishment of causality. The symptoms of Long COVID can fluctuate over time, and the participants were assessed at a single time point1. The sample size may be underpowered for some analyses, particularly due to the limited number of participants without fatigue and dyspnea. We excluded individuals with severe functional limitations and dyspnea for safety reasons. Including these patients might have strengthened the associations between adverse physical symptoms of Long COVID and movement behavior/functional capacity. Additionally, patients who cannot live alone due to severe functional limitations17 or who struggle with leaving the house or dressing due to shortness of breath18 would face significant challenges in participating in the research procedures. We also excluded individuals who engage in regular physical exercise. Lastly, our sampling was voluntary and non-probabilistic. Therefore, our results should be interpreted with caution. However, the novel results of this study may provide valuable clinical insights into the relationship between adverse physical symptoms, movement behaviors, and functional capacity in individuals with Long COVID.
In conclusion, fatigue and post-exertional malaise were negatively associated with physical activity, while dyspnea showed a negative association with functional capacity in individuals with Long COVID. These findings should be taken into consideration in rehabilitation programs delivered for this population.
Data availability
The raw data is available at supplementary material two.
References
NICE. COVID-19 Rapid Guideline: Managing the Long Term Effects of Covid-19. (2021).
Shen, Q. et al. COVID-19 illness severity and 2-year prevalence of physical symptoms: An observational study in Iceland, Sweden, Norway and Denmark. Lancet Reg. Health Eur. 35, 100756. https://doi.org/10.1016/j.lanepe.2023.100756 (2023).
Huang, Q. et al. One-year temporal changes in long COVID prevalence and characteristics: A systematic review and meta-analysis. Value Health 26(6), 934–942. https://doi.org/10.1016/j.jval.2022.11.011 (2023).
O’Mahoney, L. L. et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine. 55, 101762. https://doi.org/10.1016/j.eclinm.2022.101762 (2023).
Heidemann, C. et al. Long-term health consequences among individuals with SARS-CoV-2 infection compared to individuals without infection: Results of the population-based cohort study CoMoLo Follow-up. BMC Public Health 23(1), 1587. https://doi.org/10.1186/s12889-023-16524-8 (2023).
McAlister, F. A. et al. The risk of death or unplanned readmission after discharge from a COVID-19 hospitalization in Alberta and Ontario. Can. Med. Assoc. J. 194(19), E666–E673. https://doi.org/10.1503/cmaj.220272 (2022).
Huerne, K. et al. Epidemiological and clinical perspectives of long COVID syndrome. Am. J. Med. Open. 9, 100033. https://doi.org/10.1016/j.ajmo.2023.100033 (2023).
Aschman, T. et al. Post-COVID exercise intolerance is associated with capillary alterations and immune dysregulations in skeletal muscles. Acta Neuropathol. Commun. 11(1), 193. https://doi.org/10.1186/s40478-023-01662-2 (2023).
Weldon, E. J. et al. Mechanisms and severity of exercise intolerance following COVID-19 and similar viral infections: A comparative review. Cureus. 15, e39722. https://doi.org/10.7759/cureus.39722 (2023).
Koleničová, V. et al. A review article on exercise intolerance in long COVID: Unmasking the causes and optimizing treatment strategies. Med. Sci. Monit. 29, e941079-1-e941079-9. https://doi.org/10.12659/MSM.941079 (2023).
Wright, J., Astill, S. & Sivan, M. The relationship between physical activity and long COVID: A cross-sectional study. Int. J. Environ. Res. Public Health. 19(9), 5093. https://doi.org/10.3390/ijerph19095093 (2022).
Hennigs, J. K. et al. Respiratory muscle dysfunction in long-COVID patients. Infection. 50(5), 1391–1397. https://doi.org/10.1007/s15010-022-01840-9 (2022).
Jimeno-Almazán, A. et al. Relationship between the severity of persistent symptoms, physical fitness, and cardiopulmonary function in post-COVID-19 condition. A population-based analysis. Intern. Emerg. Med. 17(8), 2199–2208. https://doi.org/10.1007/s11739-022-03039-0 (2022).
Núñez-Cortés, R. et al. Feasibility of the 30 s sit-to-stand test in the telehealth setting and its relationship to persistent symptoms in non-hospitalized patients with long COVID. Diagnostics. 13(1), 24. https://doi.org/10.3390/diagnostics13010024 (2022).
Dowd, K. P. et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: A DEDIPAC study. Int. J. Behav. Nutr. Phys. Act. 15(1), 15. https://doi.org/10.1186/s12966-017-0636-2 (2018).
Freedson, P. S., Melanson, E. & Sirard, J. Calibration of the computer science and applications, Inc. accelerometer. Med. Sci. Sports Exerc. 30(5), 777–781. https://doi.org/10.1097/00005768-199805000-00021 (1998).
Machado, F. V. C. et al. Construct validity of the Post-COVID-19 Functional Status Scale in adult subjects with COVID-19. Health Qual. Life Outcomes. 19(1), 1–11. https://doi.org/10.1186/s12955-021-01691-2 (2021).
Kovelis, D. et al. Validação do Modified Pulmonary Functional Status and Dyspnea Questionnaire e da escala do Medical Research Council para o uso em pacientes com doença pulmonar obstrutiva crônica no Brasil. J. Bras. Pneumol. 34(12), 1008–1018. https://doi.org/10.1590/S1806-37132008001200005 (2008).
Cho, H. J. et al. Cross-cultural validation of the Chalder Fatigue Questionnaire in Brazilian primary care. J. Psychosom. Res. 62(3), 301–304. https://doi.org/10.1016/j.jpsychores.2006.10.018 (2007).
Cotler, J., Holtzman, C., Dudun, C. & Jason, L. A brief questionnaire to assess post-exertional malaise. Diagnostics. 8(3), 66. https://doi.org/10.3390/diagnostics8030066 (2018).
Fernández-de-las-Peñas, C., Navarro-Santana, M., Plaza-Manzano, G., Palacios-Ceña, D. & Arendt-Nielsen, L. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived severe acute respiratory syndrome coronavirus 2 infection: A systematic review and meta-analysis. Pain. 163(7), 1220–1231. https://doi.org/10.1097/j.pain.0000000000002496 (2022).
Choi, L., Liu, Z., Matthews, C. E. & Buchowski, M. S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sport Exerc. 43(2), 357–364. https://doi.org/10.1249/MSS.0b013e3181ed61a3 (2011).
Sasaki, J. et al. Orientações para utilização de acelerômetros no Brasil. Rev Bras Atividade Física Saúde. 22(2), 110–126. https://doi.org/10.12820/rbafs.v.22n2p110-126 (2017).
Issues S, Test MW, Equipment R, Preparation P. American Thoracic Society ATS Statement : Guidelines for the Six-Minute Walk Test. 166, 111–117https://doi.org/10.1164/rccm.166/1/111 (2002).
Britto, R. R. et al. Reference equations for the six-minute walk distance based on a Brazilian multicenter study. Brazilian J. Phys. Ther. 17(6), 556–563. https://doi.org/10.1590/S1413-35552012005000122 (2013).
Rikli, R. E. & Jones, C. J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act. 7(2), 129–161. https://doi.org/10.1123/japa.7.2.129 (1999).
Furlanetto, K. C. et al. Reference values for 7 different protocols of simple functional tests: A multicenter study. Arch. Phys. Med. Rehabil. 103(1), 20-28.e5. https://doi.org/10.1016/j.apmr.2021.08.009 (2022).
Andrade, C. Z scores, standard scores, and composite test scores explained. Indian J. Psychol. Med. 43(6), 555–557. https://doi.org/10.1177/02537176211046525 (2021).
Salive, M. E. Multimorbidity in older adults. Epidemiol. Rev. 35(1), 75–83. https://doi.org/10.1093/epirev/mxs009 (2013).
Gil, S. et al. Post-acute sequelae of SARS-CoV-2 associates with physical inactivity in a cohort of COVID-19 survivors. Sci. Rep. 13(1), 215. https://doi.org/10.1038/s41598-022-26888-3 (2023).
Makihara, A. et al. The Association between fatigue and physical activity in patients hospitalized with subacute stroke. Top. Stroke Rehabil. https://doi.org/10.1080/10749357.2023.2293337 (2023).
Sánchez-Sánchez, M. L. et al. Association of barriers, fear of falling and fatigue with objectively measured physical activity and sedentary behavior in chronic stroke. J. Clin. Med. 10(6), 1320. https://doi.org/10.3390/jcm10061320 (2021).
Gil, S. et al. Acute muscle mass loss predicts long-term fatigue, myalgia, and health care costs in COVID-19 survivors. J. Am. Med. Dir. Assoc. 24(1), 10–16. https://doi.org/10.1016/j.jamda.2022.11.013 (2023).
Joli, J., Buck, P., Zipfel, S. & Stengel, A. Post-COVID-19 fatigue: A systematic review. Front. Psychiatry. https://doi.org/10.3389/fpsyt.2022.947973 (2022).
Parker, M. et al. Effect of using a structured pacing protocol on post-exertional symptom exacerbation and health status in a longitudinal cohort with the post-COVID-19 syndrome. J. Med. Virol. https://doi.org/10.1002/jmv.28373 (2023).
Tryfonos, A. et al. Functional limitations and exercise intolerance in patients with post-COVID condition. JAMA Netw. Open. 7(4), e244386. https://doi.org/10.1001/jamanetworkopen.2024.4386 (2024).
Azadi, M. et al. Prevalence, pattern, and correlates of polypharmacy among iranian type II diabetic patients: Results from pars cohort study. Arch. Iran Med. 24(9), 657–664. https://doi.org/10.34172/aim.2021.94 (2021).
Acknowledgements
The authors acknowledge the graduate student for valuable contribution in the data collection and all patients who agreed to participate. We also acknowledge Lucas Duarte for figure abstract designing. This study received funding from the Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) through the EDITAL IMPACTOS DA PANDEMIA #0057/2022.
Author information
Authors and Affiliations
Contributions
F.J.R-S. and Y.A.F. were a major contributor in developing the methods and writing the manuscript. L.M.G., J.C.B.L.P., R.S.D. and A.M.G. helped to plan the research, validate the methods and draft the manuscript. F.D.A, C.E.S.G. and J.C.B.L.P. helped with data acquisition. C.R.R, R.M.R-T and E.C.C. supervised the research and helped to draft the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rosa-Souza, F.J., Freire, Y.A., Galliano, L.M. et al. Association of physical symptoms with accelerometer-measured movement behaviors and functional capacity in individuals with Long COVID. Sci Rep 14, 20652 (2024). https://doi.org/10.1038/s41598-024-71589-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71589-8
- Springer Nature Limited