Abstract
The inflammation and coagulopathy during coronavirus disease (COVID-19) impairs the efficiency of the current stroke treatments. Remote ischaemic conditioning (RIC) has shown potential in recent years to protect the brain and other organs against pathological conditions. This study aimed to evaluate the efficiency of RIC in brain infarct size using TTC staining and lung injury reduction by H&E staining during the hyper-inflammatory response in rats. The inflammation and coagulopathy were assessed by sedimentation rate, haematocrit, systemic oxidative stress and clotting time. Moreover, we observed changes in the cytokine profile. The results of the first part of the experiment showed that the inflammation and lung injury are fully developed after 24 h of intratracheal LPS administration. At this time, we induced focal brain ischaemia and examined the effect of RIC pre- and post-treatment. Our results showed that RIPre-C reduced the infarct size by about 23%, while RIPost-C by about 30%. The lung injury was also reduced following both treatments. Moreover, RIC modulated systemic inflammation. The level of chemokines CINC-1, LIX and RANTES decreased after 24 h of post-ischaemic reperfusion in treated animals compared to non-treated. The RIC-mediated decrease of inflammation was reflected in improved sedimentation rate and hematocrit, as well as reduced systemic oxidative stress. The results of this work showed neuroprotective and lung protective effects of RIC with a decrease in inflammation response. On the basis of our results, we assume that immunomodulation through the chemokines CINC-1, LIX, and RANTES play a role in RIC-mediated protection.
Similar content being viewed by others
Introduction
In 2019, stroke was the second leading cause of death and disability worldwide1. During the past 3 decades, in absolute terms, the global stroke incidence has increased by 70%, its mortality has increased by 43% and disability due to stroke has increased by 32%1. In 2019, the five leading risk factors for stroke were high systolic blood pressure, high body-mass index, high fasting plasma glucose concentrations, ambient particulate matter pollution and smoking1.
The novel coronavirus disease (COVID-19) has become another risk factor for stroke since 2020, with acute ischaemic stroke being reported in 4.6% of patients with COVID-192. Infected patients may develop significant coagulopathy, which leads to thromboembolic complications like stroke, peripheral artery thrombosis, deep vein thrombosis, pulmonary embolism, myocardial infarction, ischaemic stroke and venous sinus thrombosis3. Histopathological analysis of a COVID-19 positive patient´s brain after stroke revealed hypoxic neurons, significant oedema from the underlying ischaemic insult, fibrin thrombi in small vessels and fibroid necrosis of the vascular wall without any signs of vasculature inflammation4. The authors suggested that the cerebrovascular thromboembolic events in these patients may be related to hypercoagulability and coagulation cascade activation due to the release of inflammatory markers and cytokines4. Microthrombi within the vessels were more consistent with a systemic inflammatory response-mediated mechanism, probably related to elevated serum inflammatory markers such as D-dimer and fibrinogen3,4.
Coagulopathy of COVID-19 affects the efficiency of the primary treatment of acute ischaemic stroke, thrombolysis using recombinant tissue plasminogen activator (rt-PA), because the effect of rt-PA depends on haemostasis factors affecting clot structure5. Wright et al. observed a complete lack of fibrinolysis in 57% of severe COVID-19 patients through high D-dimer and unsuccessful clot lysis at 30 min by means of thromboelastography6,7. In agreement with this, Heinz et al. found more resistance to fibrinolysis in critically ill COVID-19 patients than in healthy controls7,8. Moreover, haemorrhagic transformation of ischaemic stroke was observed in some cases9. The demand for the development of new therapies to alleviate the extent of the patient's overall disability after a stroke is currently even more relevant.
The widespread increase in SARS CoV-2 variants has exhibited increased transmissibility, causing more severe disease, evasive immune properties, impaired neutralization by antibodies from vaccinated individuals or convalescence sera, and reinfection (reviewed in10), and elicited different degrees in host's response across SARS CoV2 variants (reviewed in11). Despite the variant, infection dynamics of SARS-CoV-2 and the antigenic shifts to evade host immunity are highly dependent on the host characteristics that influence the selection pressure within an endemic setting12. However, from the clinical point of view, the progress from the infection to the evolution of severe disease remains crucial regardless of the virus variant subtype. Focusing on animal models, LPS-induced lung injury and systemic inflammation is widely used to simulate severe COVID-19 infection in rodents13. However, the pathophysiology of this disease shares similarities with numerous viral or bacterial infections. Acute respiratory distress syndrome (ARDS) is a severe form of acute lung injury such as pneumonia and infection caused by bacteria or viruses. Activation of toll-like receptor-4 (TLR-4) with LPS during ARDS induces leukocyte recruitment to the lungs, pro-inflammatory cytokine release, and consequent lung injury induction, which shares many similarities with ARDS after SarS-CoV-2 infection. In this preliminary study, we decided to use this model to investigate the effect of RIC in infarct size reduction during hyper-inflammation in general. This experimental model offers a versatile platform for investigating a wide range of infections therefore results of this study should be useful also for simulating the outcomes of other diseases characterized by hyper-inflammatory response of the host organism14.
Remote ischaemic conditioning (RIC) of the limbs has shown potential in recent years to protect the brain and other organs against pathological conditions. RIC is the application of brief, nonlethal bouts of an ischaemic challenge before (pre-conditioning), during (per-conditioning), or after (post-conditioning) severe stress in a distant organ14. It represents a non-invasive, safe and cheap therapeutic approach, because sublethal limb ischaemia can be induced, for example, by a pressure gauge cuff, which could easily be translated into clinical practice. Based on current knowledge, RIC signaling from the limb to a remote internal organ suggests the involvement of a neuro-humoral pathway leading to systemic response occurring in all organs tested so far15. As reviewed in16, neuronal and humoral components involved in the signal transfer act together on three different levels. Stimulus (first) level involves direct activation of peripheral sensory nerves and/or releasing humoral factors which subsequently activate the second level—peripheral sensory nerves. Peripheral sensory afferent nerves project into autonomic centers of the central nervous system and activate efferent vagal nerves. On the level of the target organ, neuronal activation of receptors in the target organ could be accompanied by the activation of the intrinsic nervous system of the target organ via released humoral factors and/or by the receptor-dependent or -independent response of the specific parenchymal cells of the target organ. The protective effect of RIC might be mediated via the regulation of plasma cytokines as well as changes in cell surface properties of immune cells (e.g. Tie-2 and CCR2) indicating the important role of humoral factors in RIC action17.
Our results showed that remote conditioning was an effective therapy for the reduction of infarct volume in the experimental model of focal ischaemia18. The question is whether RIC could be effective during the hyper-inflammatory reaction, which is an important factor for many bacterial and viral infections, COVID-19 included. Further studies showed that RIC modulates the overall immune cell number as well as the release of anti-inflammatory cytokines. A reduction of pro-inflammatory cytokines after remote conditioning has been confirmed in a model of endotoxaemia (induced by lipopolysaccharide administration) as well as models of myocardial and brain infarction19. Ischaemic postconditioning in the septic shock model was also shown to attenuate increases in systemic inflammation and neutrophil accumulation20. Moreover, hind limb preconditioning was partially protective against indirect inflammation-mediated lung injury21. This study aimed to evaluate the efficiency of RIC in the role of a preventive agent, as well as early post-stroke therapy during the hyper-inflammatory response, which represents an increased risk factor of stroke in COVID-19 positive patients, as well as the impaired condition for treatment. For the simulation of these conditions typical for COVID-19 disease, a model of intratracheal LPS administration was used. Considering the systemically mediated protection of RIC of the whole organism, we also monitored the positive effect of RIC on the lung injury and systemic inflammation induced by LPS.
Methods
Experimental scheme
In experiments adult male albino Wistar rats were used (bred at certified vivarium of Institute of Neurobiology SAS originated from Velaz, Czech Republic, 6 mounts old, weighing 300–330 g). Rats were maintained on a 12 h light/dark cycle and given food and water ad libitum. Food was withdrawn one day before surgery.
The experiment was divided in the two parts (Fig. 1A,B). First, the model of intratracheal LPS administration was characterised (Fig. 1A). The animals were divided into three groups: the rats of the control group received saline solution by intratracheal instillation (n = 6), while the remaining rats received LPS (2.5 mg/kg) and survived for 24 h (LPS24h group, n = 6) and 48 h (LPS48h group, n = 6). In these intervals, lung injury induced by LPS intoxication was examined. The blood was sampled after 6, 24 and 48 h of LPS administration. The blood collected before LPS administration is referred to as the control value. Cytokine profile, haematology parameters and systemic oxidative stress influenced by the hyper-inflammatory response was monitored in this experiment.
Scheme of experiment. (A) Characterisation of the hyper-inflammation model induced by the intratracheal administration of LPS. Blood inflammatory parameters were evaluated during the acute phase of LPS intoxication (after 6 h) and sub-acute phase (after 24 and 48 h). Lung injury was examined after 24 and 48 h. (B) Investigation of the effect of RIC treatment on the infarct size, lung injury and systemic inflammation after the induction of hyper-inflammation. Transient focal brain ischaemia with duration 90 min (I90) was induced after 24 h of LPS intoxication and the rats were sacrificed after 24 h of reperfusion. RIC treatment was applied 1 h before (RIPre-C) and 1 h after cerebral ischaemia (RIPost-C) by hind-limb ischaemia with duration 5 min in three cycles (LI3 × 5). RIC-mediated changes in blood inflammatory parameters were examined in early (3 h of reperfusion) and delayed (24 h of reperfusion) phases of post-ischaemic reperfusion. Infarct size and lung injury was determined after 24 h of reperfusion. I90—transient focal brain ischemia lasting 90 min, LI3 × 5 limb ischemia lasting 5 min in three cycles, LPS lipopolysaccharide, RIPre-C remote ischaemic preconditioning, RIPost-C remote ischaemic postconditioning, R3h 3 h of reperfusion, R24h 24 h of reperfusion.
The main idea of the second part of the experiment was to examine the effect of remote conditioning on brain and lung injury during the hyper-inflammatory response (Fig. 1B). The rats were divided into three groups, all of which underwent focal cerebral ischaemia 24 h after the administration of LPS. The first group contains those rats without RIC treatment (the ischaemia group, n = 6), while the remaining rats were conditioned before (RIPre-C group, n = 6) and after cerebral ischaemia induction (RIPost-C group, n = 6). The blood was sampled at 3 h (R3h) and 24 h after reperfusion (R24h). The blood collected before LPS administration is referred to as the control value. The impact of RIC on the inflammatory parameters in blood was monitored at R3h and R24h. The infarct size and lung injury was examined one day of post-ischemic reperfusion.
Rat models
LPS administration
Severe disease phenotypes of COVID-19 were induced by single LPS exposure, which was injected directly to the lungs by intratracheal instillation. The rats were anaesthetised with 4% isoflurane in the anaesthetic cage and anaesthesia was maintained using 1.5% isoflurane during surgery. The rats were fixed supine on a platform inclined at 45°. The bolus of 50 µl of LPS (2.5 mg/kg) was aspirated with a syringe into the distal end of a cannula, which was then introduced into the tracheal opening and the solution was injected into the lungs.
Remote ischaemic conditioning
Right hind-limb ischaemia was induced by placing an elastic rubber band tourniquet on the proximal part of the limb for 5 min, followed by a 5-min period of reperfusion according to a published protocol22. The procedure was performed under light isoflurane anaesthesia (0.5%) in three cycles. The treatment was induced 1 h before (remote ischaemic preconditioning—RIPre-C) and 1 h after (remote ischaemic postconditioning—RIPost-C) focal cerebral ischaemia induction. Non-treated rats underwent the same procedure without the tourniquet application.
Transient focal ischaemia induction
For the induction of transient focal cerebral ischaemia (stroke), right middle cerebral artery occlusion (MCAO) was used, as described by23. Briefly, the rats were anaesthetised with 4% isoflurane in the anaesthetic cage, which was maintained during surgery with 1.5% isoflurane. The operation was performed under an operating microscope. In the first step, the right common carotid artery (CCA), the right external carotid artery (ECA) and the right internal carotid artery (ICA) were isolated. The right ECA was tied by the node at the distal end permanently and then a filament was inserted and pushed up the ICA until light resistance was felt. MCAO suture for rats (331–400 g, tip 0.41–0.42 mm; RWD Life Science Co, Ltd. China) was inserted approximately 19–20 mm from the carotid bifurcation to effectively block the middle cerebral artery (MCA). The severity of MCA occlusion was confirmed by measurement of local cerebral blood flow using a laser-Doppler flow meter (PeriFlux System 5000; Perimed AB, Jarfalla, Sweden; Fig. 3). A 407 probe with an adequate holder was situated on the skull over the MCA (5 mm lateral and 1 mm posterior to bregma). Only rats with blood flow reduced by more than 80% were used in the experiments. The duration of filament insertion was 90 min, after which it was removed.
Neurological deficit was assessed in each animal on a numerical scale of 0–4 after 1 h and one day of post-ischaemic reperfusion. The scoring system based on24 was used: (0) no detectable deficits; (1) forelimb flexion and torso turning to the contralateral side when lifted by the tail; (2) the same behaviour as grade 1 and decreased resistance to a lateral push; (3) the same behaviour as grade 2 with unilateral circling; and (4) no spontaneous walking and a depressed level of consciousness. Rats with a neurological deficit lower than grade 2 were excluded from the study.
Haematology tests and animal body temperature
Temperature
The change in body temperature of the rats was measured using the rectal thermometer (ATC1000, World precision instruments Sarasota, FL) 6, 12 and 24 h after the LPS intoxication and following 3 and 24 h of post-ischaemic reperfusion.
Clotting time
This test measures the time it takes for a clot to form in a blood sample. A non-heparinised capillary tube was filled with blood. The capillary tube was broken and slowly separated in 10 s intervals, until the blood formed a continuous thread-like clot between the broken ends of the tube. The time from capillary filling to the appearance of the clot thread is the clotting time expressed in seconds (s).
Erythrocyte sedimentation rate (ESR)
Erythrocyte sedimentation rate is a blood test that can indicate and monitor an increase in inflammatory activity. The sample of anti-coagulated whole blood was allowed to stand in the vertical tube for 1 h. The red blood cells slowly fell to the bottom due to the influence of gravity. The test result depends on the amount of plasma at the top of the tube, measured after 1 h. The result is reported in millimetres per hour (mm/hr).
Haematocrit
A haematocrit test measures the proportion of red blood cells in the blood. A heparinised capillary tube was filled with blood and centrifuged (12,000 rpm, 10 min). Haematocrit was estimated by calculating the ratio of the column of packed erythrocytes to the total length of the sample in the capillary tube and expressed as a percentage of the blood cells (% of blood cells).
Systemic oxidative stress
DNA damage in circulating lymphocytes was assessed by single-cell gel electrophoresis (SCGE) according to Singh et al.25, with minor modifications. Isolated lymphocytes were mixed with 1% low-melting-point agarose in PBS (pH 7.4) and added to microscope slides precoated with 1% normal-melting-point agarose in PBS (pH 7.4). The suspension was covered with a coverslip and allowed to solidify in a refrigerator for 20 min. The coverslip was removed and the slides were submerged in lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1% Triton X-100 and 10% DMSO, pH 10) for 1 h. The slides were then placed in the electrophoresis buffer (5 M NaOH, 200 mM Na2EDTA, pH 13) for 20 min. To assess single-strand breaks (SSBs) in lymphocyte DNA, an alkaline pH is necessary to unwind the DNA. Electrophoresis was carried out using the same solution for 25 min at 25 V. After electrophoresis, the slides were neutralised with neutralisation buffer (400 mM Tris, pH 7.5, 15 min). The above-mentioned steps were carried out in a refrigerator (4 °C). Slides were stained with SYBR GOLD nucleic acid gel stain directly before being imaged under a fluorescence microscope (Olympus BX51, excitation at 485 nm, emission at 520 nm) equipped with a camera (Olympus DP50). Comet micrographs (Olympus DP image software) were processed using the CometScore™ 1.5 image analysis system (TriTek Corp., USA). Lymphocyte DNA damage was examined by the parameter ‘% DNA in tail’ (100% of cell fluorescence intensity minus the intensity of the % DNA in the head). For each individual sample, two slides were prepared and cells were randomly selected for analysis (100 cells per sample).
Cytokine profile
The cytokine profile was assessed using a membrane-based sandwich immunoassay Proteome Profiler RatCytokine Array Kit (Panel A, R&D Systems). Here, 200 µl of plasma from five rats in each group was pooled (1000 µl total volume/group). The samples were mixed with a cocktail of biotinylated detection antibodies and incubated at room temperature for 1 h. The sample/antibody mixture was added to membranes spotted with capture antibodies and incubated overnight at 4°C. Following a wash, the streptavidin–HRP and chemiluminescent reagent mix was applied. The chemiluminescent signal was captured by a Fusion FX Chemiluminescent system (Vilber Lourmat). Pixel densities from each membrane were analysed using the ImageQuant™ TL analysis software and normalised to references spots on each membrane.
Infarct size
The brain was isolated and cut into 2 mm coronal slices. The 4th slice was immersed in a 1% solution of 2,3,5-triphenyltetrazolium chloride in PBS and incubated (37 °C, 15–20 min). After subsequent fixation in 10% formalin, the section was scanned using a high-resolution scanner (Hewlett Packard Scanjet) and images were processed using Image J software (National Institutes of Health, Bethesda, Maryland, USA) for the final infarct size calculation. The non-ischaemic hemisphere (contralateral), ischaemic hemisphere (ipsilateral) and infarct area of each brain section were measured in a blinded manner and the average infarct area was calculated by the formula: (infarct area on the anterior surface + infarct area on the posterior surface)/2. The infarct volume (%), corrected to compensate for brain oedema, was calculated by applying the formula [contralateral hemisphere volume—(ipsilateral hemisphere volume—infarct volume)]/contralateral hemisphere volume × 10026.
Lung injury
Lung oedema
The lobe of the right lung was used to determine lung oedema by calculating the wet/dry weight ratio. The lobe was weighed immediately after excision and then left to dry for 1 day in an incubator set at 70 °C before being weighed again. The oedema was expressed as a weight ratio of the wet and dry lung section.
Alveolar septa thickening
The left lobe of the lung was used for the histological analysis. The tissue was fixed in formalin for 3 days, cut into 5 µm thick sections and stained with haematoxylin and eosin (H&E).
An investigator blinded to group settings screened and analysed 25–30 fields (20 × objective magnification) covering the entire slide of each sample using ImageJ software. The percentage of tissue area (thickness of alveolar septa) per field was quantified for all screened images. The tissue (red signal) areas were set as hue (0–255), saturation (0–255) and intensity (212–255) and the alveoli (white signal) areas were set as hue (0–255), saturation (0–255) and intensity (0–211)27.
Results
Course of the LPS intoxication-mediated changes
In the first part of the experiment, the model of hyper-inflammation induced by LPS was characterised. We monitored the systemic response on LPS intoxication and plasma cytokine profile changes during the hyper-acute phase (6 h after the administration of LPS) and two time points of the acute stage (24 h and 48 h after LPS intoxication). Subsequently, after inflammation development during the acute stage, an indirect inflammation-mediated lung injury was determined.
Systemic response on the LPS administration
Although some of the blood parameters started to rise 6 h after LPS intoxication, the complete systemic response reflected by significant alterations of all parameters became visible at 24 h (Fig. 2). At this time, we observed a clotting time which was decreased by half as well as decreased haematocrit by about 15% compared to the control values. The initiation of inflammatory processes was reflected by a 2.5-fold increase in erythrocyte sedimentation rate. Systemic oxidative stress related to inflammatory changes increased more than 8 times over the control (5.7 ± 2.2% DNA in tail). The temperature of rats was not affected by LPS administration during the experiment.
Graphical evaluation of systemic response to LPS intoxication. Changes in temperature, clotting time, haematocrit, sedimentation rate and oxidative stress after 6, 24 and 48 h of LPS intoxication. Control value is set to 100%. The mean ± SD. (* = p ˂ 0.01, ** = p ˂ 0.001, *** = p ˂ 0.0001 compared to Control) LPS—lipopolysaccharide, ESR—erythrocyte sedimentation rate.
Plasma cytokine profile after the LPS intoxication
We used a rat cytokine array for the parallel determination of relative levels of selected cytokines and chemokines following LPS intoxication. The hyper-acute phase (6 h) of intoxication was characterised by the rose of the three pro-inflammatory cytokines/chemokines CINC-1, LIX and ICAM-1 (Fig. 3A,B). The acute stage (24 h after LPS administration) is characterised by elevated ICAM-1 and by the transient increase in CXCL7. On the other hand, the drop of CINC-1, LIX and RANTES was detected at this time. The baseline value of all observed cytokines/chemokines was reached after 48 h of LPS intoxication. The level of LIX and RANTES dropped even under the control value at this time.
Cytokine profile in plasma after LPS local exposure. (A) Fold changes in cytokine/chemokine levels in plasma after 6, 24 and 48 h of LPS intoxication compared to control values. (B) Relative levels of cytokines/chemokines expressed in arbitrary units. The mean ± SD. (* = p ˂ 0.05, **** = p ˂ 0.0001 increase compared to Control; &&&& = p ˂ 0.0001 decrease compared to Control) LPS lipopolysaccharide.
Lung injury induced by the administration of LPS
H&E staining was used to reveal histological changes of the lung. Typical signs for lung injury are thickening of the alveolar septa and oedema. LPS intoxication caused an increase in alveolar septa thickness after 24 h (Fig. 4A,B), which was also significant during the next 24 h (Fig. 4A,B).
Lung injury induced by local LPS exposure after 24 and 48 h. (A) Representative pictures of H&E staining of lungs after 24 and 48 h of LPS intoxication. Bar = 100 µm. (B) Graphical evaluation of the alveolar septa thickening expressed as % of tissue area. (C) Oedema in the lungs expressed as a weight ratio of wet and dry lung section. Mean ± SD. (** = p ˂ 0.01, *** = p ˂ 0.001 compared to Control) LPS lipopolysaccharide.
We detected oedema in the lung one day after local exposure to lipopolysaccharide (4.8 ± 0.2 w/d ratio), which was subsequently reduced almost to control values during the next 24 h (4.5 ± 0.3 w/d ratio; Control 4.3 ± 0.2 w/d ratio) (Fig. 4C).
RIC-mediated changes in the condition of hyper-inflammation
The results of the first part of the experiment showed that the inflammation and lung injury induced by LPS is fully developed at 24 h after administration. At this time, we induced brain ischaemia and examined the impact of RIC on brain and lung injury during the hyper-inflammatory response. Moreover, we investigated the modulation of inflammation by RIC during the early (3 h) and delayed phases (24 h) of post-ischaemic reperfusion.
Systemic response on RIC after focal ischaemia during hyper-inflammation state
The temperature was not affected by LPS exposure (38 °C). On the other hand, brain ischaemia mediated temperature increases after 3 h (above 39 °C). Surprisingly, only RIC pre-treatment abolished post-ischaemic hyperthermia (Fig. 5A).
Systemic response on RIC after focal ischaemia during hyper-inflammation. Changes in (A) temperature, (B) sedimentation rate, (C) haematocrit, D) clotting time and E) systemic oxidative stress mediated by RIC during the early (3 h–R3h) and delayed (24 h–R24h) phases of post-ischaemic reperfusion. Cerebral ischaemia was induced after 24 h of LPS intoxication. The blood collected before LPS administration is referred to as the control value. The results of temperature, haematocrit, and clotting time are expressed in %. Control value is set to 100%. The results of sedimentation rate are expressed in mm/hour and DNA damage of peripheral lymphocytes in % DNA in tail. Mean ± SD (* = p ˂ 0.05, ** = p ˂ 0.01, **** = p ˂ 0.0001, RIC compared to Ischaemia; & = p ˂ 0.05, && = p ˂ 0.01, &&& = p ˂ 0.001, &&&& = p ˂ 0.0001, Ischaemia compared to Control) LPS lipopolysaccharide, RIPre-C remote ischaemic preconditioning, RIPost-C remote ischaemic postconditioning, R3h 3 h of reperfusion, R24h 24 h of reperfusion.
RIC, regardless of the application time, improved the erythrocyte sedimentation rate by about 50% after 3 h of reperfusion (Ischaemia 3.1 ± 1.5 mm/h; RIPre-C 1.4 ± 0.4 mm/h; RIPost-C 1.5 ± 0.7 mm/h) and about 28% (RIPre-C 4.9 ± 1.6 mm/h; RIPost-C 5 ± 1.3 mm/h) after 24 h of reperfusion compared to ischaemic animals (6.8 ± 0.6 mm/h) (Fig. 5B).
Haematocrit dropped by an additional 15% compared to the LPS-induced reduction at 3 h following ischaemia (Control value 62.3 ± 4.1% of blood cells; LPS24h 52.7 ± 5.1% of blood cells; Ischaemia 43.5 ± 6.2% of blood cells) (Fig. 5C). Hind limb treatment preserved the control values of haematocrit after 3 h of reperfusion (RIPre-C 62.9 ± 4.1% of blood cells; RIPost-C 58.2 ± 4% of blood cells), but not after 24 h, when we detected a decrease of about 12% in preconditioned animals (58 ± 8.6% of blood cells) and about 19% in post-conditioned animals (50 ± 1.7% of blood cells).
Clotting time reduced by half after 24 h of LPS exposure but was not affected by brain ischaemia (Fig. 5D). RIC treatment transiently improved clotting time in the early stage of post-ischaemic reperfusion; however, the loss of its efficiency was observed after one day.
Remote conditioning applied before as well as after the brain ischaemia caused a reduction in the systemic oxidative stress (Fig. 5E). The ischaemia induction caused an increase in DNA damage of peripheral lymphocytes after 3 h of reperfusion (9 ± 2.7% DNA in tail) up to one day (15.5 ± 4.7% DNA in tail). The systemic response to ischaemia in LPS-treated animals was completely abolished in RIC-conditioned groups.
RIC-mediated changes in cytokine profile after cerebral ischaemia during hyper-inflammatory response
In this part of the experiment, we focused on cytokine/chemokine levels, which were influenced by LPS intoxication in the first instance; we observed changes induced by ischaemia induction and RIC treatment. Compared to ischaemia, RIC treatment mediated changes in the cytokine profile, but in a time- and treatment-specific manner (Fig. 6A,B). As described above, the pre- or post-conditioning of transient focal brain ischemia subjected animals results in almost identical systemic responses to treatments, showing the early (3 h of reperfusion) and delayed (24 h of reperfusion) reply. Therefore, the RIC effect on cytokine profile in this experiment was determined based on 1) similarities in RIC-mediated changes applied as pre- and post-conditioning treatments and 2) the time-dependent response of RIC treatment (3 and 24 h of reperfusion).
RIC-mediated changes in cytokine profile after cerebral ischaemia during the hyper-inflammatory response. Changes in cytokine/chemokine levels after RIC during the (A) early (3 h–R3h) and (B) delayed (24 h–R24h) phase of post-ischaemic reperfusion compared to non-treated animals. Relative levels of C) CINC-1, D) LIX and E) RANTES expressed in arbitrary units. Mean ± SD (* = p ˂ 0.05, ** = p ˂ 0.01, *** = p ˂ 0.001, RIC compared to Ischaemia; αα = p ˂ 0.01, ααα = p ˂ 0.001, αααα = p ˂ 0.0001, R3h compared to R24h) LPS lipopolysaccharide, RIPre-C remote ischaemic preconditioning, RIPost-C remote ischaemic postconditioning, R3h 3 h of reperfusion, R24h 24 h of reperfusion.
On the basis of these specifications, we selected the chemokines CINC-1, LIX and RANTES (Fig. 6C–E). At 3 h of post-ischaemic reperfusion, the plasma levels of these hyper-acute chemokines increased transiently. At this time, we observed elevated levels of CINC-1 (Fig. 6C) and LIX (Fig. 6D) in RIC-treated animals; however, plasma levels of RANTES dropped in post-conditioned rats (Fig. 6E). In the acute stage of post-ischaemic reperfusion, we observed a downward trend of relative amounts of all of them. However, the reduction following the RIC treatment (pre- and post-) was meaningful. In summary, RIC applied before and after brain ischaemia influences the level of chemokines predominantly during the acute phase of post-ischaemic reperfusion.
Effect of RIC on inflammation-mediated lung injury
We observed a significant reduction of alveolar septa thickening in RIC-treated animals; while preconditioning caused a reduction of about 3.5%, postconditioning caused a reduction of up to 5% (Fig. 7A,B). We observed no changes in lung oedema after 24 h of reperfusion in animals with (RIPre-C 4.4 ± 0.6 w/d ratio; RIPost-C 4.6 ± 0.3 w/d ratio) or without RIC treatment (4.5 ± 0.1 w/d ratio) (Fig. 7C).
Effect of RIC on the lung injury induced by LPS intoxication. (A) Representative pictures of H&E-stained lungs of RIC-treated and non-treated rats after 48 h of LPS intoxication. Bar = 100 µm. (B) Graphical evaluation of alveolar septa thickening expressed as % of tissue area. (C) Oedema in the lungs expressed as a weight ratio of wet and dry lung sections. Mean ± SD. (* = p ˂ 0.05, *** = p ˂ 0.001 RIC compared to Ischaemia) RIPre-C remote ischaemic preconditioning, RIPost-C remote ischaemic postconditioning.
Neuroprotective effect of the RIC on brain injury after focal ischaemia in the condition of hyper-inflammatory response
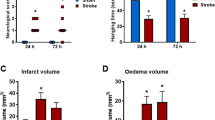
We observed the neuroprotective effect of the RIC, even under hyper-inflammatory reaction conditions (Fig. 8A,B). In preconditioned animals, we observed an infarct size reduction of about 23% (36.26 ± 15.99% of infarct volume) compared to non-treated ischaemic rats (58.77 ± 3% of infarct volume) (Fig. 8A), however with no effect on the neurological score (2.8 ± 0.5 neurological score) (Fig. 8B). Remote ischaemic postconditioning reduced the infarct area by about 30% (29.25 ± 11.19% of infarct volume) (Fig. 8A), which was also reflected in the improved neurological score (from 3 ± 0 to 1.7 ± 0.3) (Fig. 8B).
Neuroprotective effect of the RIC after focal ischaemia under the condition of hyper-inflammatory response. (A) Graphical evaluation of infarct size in RIC-treated and non-treated rats expressed as %. (B) Neurological deficit scored in the scale from 0 (no deficit) TO 4 (no spontaneous walking) according to the Bederson scoring system. Mean ± SD. (* = p ˂ 0.05, ** = p ˂ 0.01 RIC compared to Ischaemia) RIPre-C remote ischaemic preconditioning, RIPost-C remote ischaemic postconditioning.
Discussion
With the arrival of COVID-19, the severity of acute brain injury caused by stroke increased. The complications arising in positive patients affected by stroke are caused by the presence of hyper-inflammation typical of cytokine storm and coagulopathy. Related to stroke treatment, the alternative strategy based on the non-invasive stimulation of endogenous neuroprotection—RIC—has become the attractive supplementary therapy in several clinical trials. However, there are still many issues to be investigated for the successful implementation of RIC in clinics, especially related to comorbidities of stroke patients. In this work, we focused on the evaluation of the potential of remote ischaemic conditioning in infarct size reduction after stroke in a state of hyper-inflammatory response which is characteristic of many viral and bacterial infections, SARS-CoV-2 included.
Although several identified variants of the SARS CoV-2 virus presuppose the use of subtype-specific models in experimental research, the impossibility of infecting rat respiratory cells by SARS CoV-2 directs research to the use of models simulating the systemic impact of infection in the form of hyperinflammation28,29. To mimic the hyper-inflammation condition in our experiment, the model of intratracheal LPS intoxication was established. The hyper-acute phase of the model of LPS intoxication was characterised by increased levels of cytokines and chemokines in plasma, chemoattractants of the neutrophils CINC-1 and LIX and the soluble form of ICAM-1. Increased levels of sICAM-1 remained elevated up to the acute stage, 24 h following the LPS application. Increased sICAM-1 plasma level is considered a marker of lung epithelial and endothelial injury and is associated with poor clinical outcomes in patients with acute lung injury30. sICAM-1 activates macrophages in the lungs and enhances lung injury31. Increased levels of chemokines like CINC-1 and LIX lead to neutrophil activation and mobilisation to the site of inflammation, which also contributes to the lung injury. Dysregulated inflammation and intense leukocyte infiltration leads to a breakdown of the alveolar-epithelial barrier, which results in pulmonary oedema32. The pulmonary oedema in an established model of hyper-inflammation in our experiment was clearly visible after the first day. Although it was not the focus of our research, the H&E staining of lung slices revealed an increase in neutrophil infiltrates in the surrounding alveolar. Consistent with Herrero et al.32, the oedema was associated with a thickening of the alveolar septa at the same time, confirming that the time point of the development of acute lung injury had been reached.
A response in systemic parameters reflecting acute inflammation was also developed following one day when the meaningful changes in sedimentation rate, haematocrit and systemic oxidative stress were recorded in presented results. Due to a close relationship between immune system activation and the haemostatic system, it is not unexpected for LPS intoxication in this study to have significantly affected the clotting time and impaired the process of coagulation. Based on the characteristics of the animal model used for our experiment, the acute stage of LPS intoxication was selected for future studies of RIC neuroprotective properties for stroke treatment under the condition of hyper-inflammation.
After the onset of the COVID-19 pandemic, there were the several studies showing the increased mortality and poor outcome of positive patients affected by stroke33. Compared with those with mild or asymptomatic COVID-19 at stroke onset, patients who have strokes while hospitalised with severe manifestations of COVID-19 have significantly elevated inflammatory markers and markers of hypercoagulability34. The study of Finck et al.35 showed that the presence of systemic inflammation in stroke patients is linked with a poor outcome and death. The hyper-inflammatory state is associated with many other viral, bacterial or autoimmune diseases, which could influence the course, outcome and treatment of strokes.
Remote ischaemic conditioning has been intensively studied in recent years due to its wide range of protection, not only against ischaemic injury in organs. Our previous results showed that remote conditioning was an effective therapy in the reduction of infarct volume in the experimental model of focal ischaemia18. However, the threshold of its efficiency for stroke treatment in connection to obesity was recently confirmed36. This highlights the need for targeted research regarding the efficiency of experimental neuroprotective strategies. Therefore, we provide the first pre-clinical evidence of RIC outcomes in the condition of hyper-inflammation, which is characteristic of SARS-CoV-2 and other viral or bacterial infection. Considering our previous results11, we can speculate that the beneficial effect of RIC reflected in the reduction of infarction following the stroke is related rather to the activation of neuroprotective mechanisms in the organism than the suppression of hyperinflammation caused by LPS administration (although it may not be strictly excluded). In this experiment, the range of infarction of LPC-injected animals after stroke with or without RIC application did not show noticeable changes compared to animals without hyper-inflammatory reactions published previously11. However, this hypothesis must be verified adequately using specified experiments.
In the present study, we confirmed the reduction in infarct size following the pre- and post-RIC treatment during hyper-inflammation; thus, we confirmed the effectiveness of this experimental therapy, even under the conditions of an inflammatory storm. The range of infarction during hyper-inflammation was reduced by more than 20% following RIC application, which is consistent with numerous animal studies of RIC efficacy in intact conditions (summarised in37). However, data from our analysis in LPS-treated animals confirm improvement in neurological outcomes solely in post-conditioned individuals. Systemic administration of LPS, a potent TLR4 ligand of bacterial origin, renders animals tolerant to injury in several models of cerebral ischemia38,39,40,41. It suggests that the LPS application in our experiment could serve as a preconditioning per se. In combination with remote ischemic conditioning as a preventive intervention or post-stroke treatment, it might affect its final phenotypic manifestation despite its neuroprotective effect. In our previous study42 the blood transfer of ischemia and RIC-treated animals was tested for its neuroprotective properties in a rat model of stroke. Although pre-treated stroke animals by the combination of two of those independent stressors (i.e. double preconditioned) have shown the most extensive reduction of brain infarction, no improvement of neurological deficit was indicated in those rats one day later. Based on our experiences, improvement in neurological deficit might appear with longer reperfusion when using Bederson’s scoring system (up to 2–3 days), thus suggesting the need to use complex neurobehavioral tests in this type of experiment to discuss intervention-related neurological impairment in a more precise way.
Moreover our results have shown that the lung tissue injury initiated by inflammatory storm was also protected by RIC and the alveolar septa thickening was significantly improved by the application of RIC. The lung oedema induced by LPS disappeared after 48 h in treated as well as non-treated animals. The meta-analysis provided by Kashiwagi et al.43 showed the beneficial effect of remote ischaemic preconditioning on lung function in patients undergoing various surgical procedures under general anaesthesia. The lung protective effect by RIC was also observed in several animal models of lung injury arising from different stressors (reviewed in14).
Modulation of the inflammatory response seems to be the key effect of RIC treatment and could be detected at several levels. There are several studies showing the reduction of pro-inflammatory cytokine levels and an increase in anti-inflammatory cytokine levels following RIC44,45. In the present study, we observed the RIC modulation of the cytokine profile, but in a time- and treatment-specific manner. This is consistent with a previous study46, in which different expression patterns of cytokines were reported according to per- and post-conditioning, suggesting the participation of different signalling pathways, but leading to a similar effect. In this paper, we observed the comparable effect of both (RIPre-C and RIPost-C) treatments on the level of CINC-1, LIX and RANTES. The expression of these chemokines decreased significantly during the delayed phase of post-ischaemic reperfusion as a consequence of RIC treatment. All RIC-modulated chemokines play a role in neutrophil activation and their mobilisation to the site of inflammation47,48,49. Decreased levels of these chemoattractants could explain the reduced lung injury after RIC due to the modulation of leukocyte infiltration which resulted in a lower pro-inflammatory response. However, a direct influence on the modulation of infarcted tissue immunoreactivity should not be excluded. The decreased potential of neutrophil migration could also be reflected in reduced systemic oxidative stress. It is well known that activated neutrophils produce reactive oxygen species (ROS) during inflammation. Neutrophil-produced ROS play a pivotal role in many inflammatory diseases, for example in organ dysfunction during sepsis50. Our previous results showed a significant reduction of systemic oxidative stress after RIC application in a model of global brain ischaemia51. The present study showed comparable results, where we observed decreased DNA damage in peripheral lymphocytes after RIPre-C and RIPost-C application. The immunomodulatory effect of RIC was reflected also in an improved sedimentation rate and haematocrit. Although the observed inflammatory parameters were positively influenced by RIC, coagulation was modulated only transiently during the early stage, when we observed the clotting time in the baseline value. Contradictory results about the coagulation process affected by RIC were also obtained in clinical trials. While Kim et al.52 observed no effect of RIC on platelet aggregability and coagulation, Gorog et al.53 conclude that RIC may reduce platelet reactivity.
Although several experimental studies showed the immunomodulatory effect of RIC, reflecting on clinical trials, the efficiency of RIC depends on the baseline and peak inflammation (reviewed in19). Individuals with chronic inflammatory disease and persistently low levels of inflammation at baseline may already be resistant to remote conditioning19,54. In the present study, using the acute hyper-inflammation model, we observed neuroprotective as well as a lung protective effect of RIC with a decrease in the inflammation response. On the basis of our results, we assume that immunomodulation plays a key role in RIC-mediated protection. We observed the influence of RIC on the cytokine/chemokine profile in plasma; however, it depended on a time- and specific-manner. We suggest that the modulation of chemokines CINC-1, LIX and RANTES in a delayed phase of post-ischaemic reperfusion represents the common pathway leading to protection mediated by remote ischaemic pre- and post-conditioning. The immunomodulatory effect of RIC predisposes this approach to the treatment of stroke in patients with ongoing inflammation, which is a typical sign of many bacterial, viral and autoimmune diseases, including COVID-19.
Data availability
Data are available from the corresponding author on request.
References
Owolabi, M. O. et al. Primary stroke prevention worldwide: Translating evidence into action. The Lancet. Public Health 7, e74–e85 (2022).
Li, Y. et al. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 5, 279–284 (2020).
Ozturk, S. COVID-19 and stroke: A neurological perspective, in Dehkharghani, S. (Ed.) Stroke, Brisbane (AU) (2021).
Patel, S. D. et al. Malignant cerebral ischemia in A COVID-19 infected patient: Case review and histopathological findings. J. Stroke Cerebrovasc. Dis.: Off. J. Natl. Stroke Assoc. 29, 105231 (2020).
Bagoly, Z., Szegedi, I., Kalmandi, R., Toth, N. K. & Csiba, L. Markers of coagulation and fibrinolysis predicting the outcome of acute ischemic stroke thrombolysis treatment: A review of the literature. Front. Neurol. 10, 513 (2019).
Wright, F. L. et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J. Am. Coll. Surg. 231, 193-203.e191 (2020).
Sayyadi, M., Hassani, S., Shams, M. & Dorgalaleh, A. Status of major hemostatic components in the setting of COVID-19: The effect on endothelium, platelets, coagulation factors, fibrinolytic system, and complement. Ann. Hematol. 102, 1307–1322 (2023).
Heinz, C. et al. Greater Fibrinolysis resistance but no greater platelet aggregation in critically Ill COVID-19 patients. Anesthesiology 134, 457–467 (2021).
Owolabi, L. F. et al. Hemorrhagic infarctive stroke in COVID-19 patients: Report of two cases and review of the literature. J. Commun. Hosp. Internal Med. Perspect. 11, 322–326 (2021).
Ramesh, S. et al. Emerging SARS-CoV-2 variants: A review of its mutations, its implications and vaccine efficacy. Vaccines 9, 1195 (2021).
Maurya, R. et al. Co-evolution of SARS-CoV-2 variants and host immune response trajectories underlie COVID-19 pandemic to epidemic transition. iScience 26, 108336 (2023).
Sarkar, R. et al. Comprehensive analysis of genomic diversity of SARS-CoV-2 in different geographic regions of India: An endeavour to classify Indian SARS-CoV-2 strains on the basis of co-existing mutations. Arch. Virol. 166, 801–812 (2021).
Tsikis, S. T. et al. Lipopolysaccharide-induced murine lung injury results in long-term pulmonary changes and downregulation of angiogenic pathways. Sci. Rep. 12, 10245 (2022).
Incognito, A. V., Millar, P. J. & Pyle, W. G. Remote ischemic conditioning for acute respiratory distress syndrome in COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 320, L331–L338 (2021).
Keevil, H., Phillips, B. E. & England, T. J. Remote ischemic conditioning for stroke: A critical systematic review. Int. J. Stroke: Off. J. Int. Stroke Soc. 19, 271–279 (2024).
Kleinbongard, P., Skyschally, A. & Heusch, G. Erratum to: Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Archiv.: Eur. J. Physiol. 469, 843 (2017).
Hummitzsch, L. et al. Effects of remote ischemic preconditioning (RIPC) and chronic remote ischemic preconditioning (cRIPC) on levels of plasma cytokines, cell surface characteristics of monocytes and in-vitro angiogenesis: A pilot study. Basic Res. Cardiol. 116, 60 (2021).
Jachova, J., Gottlieb, M., Nemethova, M., Bona, M. & Bonova, P. Brain to blood efflux as a mechanism underlying the neuroprotection mediated by rapid remote preconditioning in brain ischemia. Mol. Biol. Rep. 47, 5385–5395 (2020).
Pearce, L., Davidson, S. M. & Yellon, D. M. Does remote ischaemic conditioning reduce inflammation? A focus on innate immunity and cytokine response. Basic Res. Cardiol. 116, 12 (2021).
Kim, Y. H., Yoon, D. W., Kim, J. H., Lee, J. H. & Lim, C. H. Effect of remote ischemic post-conditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. J. Inflamm. (Lond.) 11, 16 (2014).
Kim, Y. H. et al. Remote ischemic preconditioning ameliorates indirect acute lung injury by modulating phosphorylation of IkappaBalpha in mice. J. Int. Med. Res. 47, 936–950 (2019).
Hu, S. et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 1459, 81–90 (2012).
Longa, E. Z., Weinstein, P. R., Carlson, S. & Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91 (1989).
Bederson, J. B. et al. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 17, 472–476 (1986).
Singh, N. P., McCoy, M. T., Tice, R. R. & Schneider, E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175, 184–191 (1988).
Callaway, J. K. et al. A novel, rapid, computerized method for quantitation of neuronal damage in a rat model of stroke. J. Neurosci. Methods 102, 53–60 (2000).
Tian, Z. et al. Dynamic alterations in the lung microbiota in a rat model of lipopolysaccharide-induced acute lung injury. Sci. Rep. 12, 4791 (2022).
Munoz-Fontela, C. et al. Animal models for COVID-19. Nature 586, 509–515 (2020).
Al-Ani, B. et al. Lipopolysaccharide induces acute lung injury and alveolar haemorrhage in association with the cytokine storm, coagulopathy and AT1R/JAK/STAT augmentation in a rat model that mimics moderate and severe Covid-19 pathology. Clin. Exp. Pharmacol. Physiol. 49, 483–491 (2022).
Flori, H. R., Ware, L. B., Glidden, D. & Matthay, M. A. Early elevation of plasma soluble intercellular adhesion molecule-1 in pediatric acute lung injury identifies patients at increased risk of death and prolonged mechanical ventilation. Pediatric Crit. Care Med.: J. Soc. Crit. Care Med. World Fed. Pediatric Intensive Crit. Care Soc. 4, 315–321 (2003).
Schmal, H. et al. Soluble ICAM-1 activates lung macrophages and enhances lung injury. J. Immunol. 161, 3685–3693 (1998).
Herrero, R., Sanchez, G. & Lorente, J. A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Transl. Med. 6, 32 (2018).
de Havenon, A., Zhou, L. W., Yaghi, S., Frontera, J. A. & Sheth, K. N. Effect of COVID-19 on acute ischemic stroke severity and mortality in 2020: Results from the 2020 national inpatient sample. Stroke 54, e194–e198 (2023).
Katz, J. M. et al. COVID-19 severity and stroke: Correlation of imaging and laboratory markers, AJNR. Am. J. Neuroradiol. 42, 257–261 (2021).
Finck, T. et al. Inflammation in stroke: Initial CRP levels can predict poor outcomes in endovascularly treated stroke patients. Front. Neurol. 14, 1167549 (2023).
Kotorova, K., Koncekova, J., Gottlieb, M., Bona, M. & Bonova, P. Obesity as a limiting factor for remote ischemic postconditioning-mediated neuroprotection after stroke. J. Obes. Metab. Syndr. 33, 76–87 (2024).
Torres-Querol, C., Quintana-Luque, M., Arque, G. & Purroy, F. Preclinical evidence of remote ischemic conditioning in ischemic stroke, a metanalysis update. Sci. Rep. 11, 23706 (2021).
Tasaki, K. et al. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 748, 267–270 (1997).
Rosenzweig, H. L. et al. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke 35, 2576–2581 (2004).
Hickey, E. J., You, X., Kaimaktchiev, V., Stenzel-Poore, M. & Ungerleider, R. M. Lipopolysaccharide preconditioning induces robust protection against brain injury resulting from deep hypothermic circulatory arrest. J. Thoracic Cardiovasc. Surg. 133, 1588–1596 (2007).
Marsh, B. et al. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J. Neurosci.: Off. J. Soc. Neurosci. 29, 9839–9849 (2009).
Bonova, P. & Gottlieb, M. Blood as the carrier of ischemic tolerance in rat brain. J. Neurosci. Res. 93, 1250–1257 (2015).
Kashiwagi, S. et al. Effect of remote ischemic preconditioning on lung function after surgery under general anesthesia: A systematic review and meta-analysis. Sci. Rep. 13, 17720 (2023).
Joseph, B. et al. Survival benefits of remote ischemic conditioning in sepsis. J. Surg. Res. 213, 131–137 (2017).
Chen, C. et al. Splenic responses play an important role in remote ischemic preconditioning-mediated neuroprotection against stroke. J. Neuroinflammation 15, 167 (2018).
Coral Torres-Querol, R. P., Arqué, G., Purroy, F. Remote ischemic conditioning modulates inflammatory response and metabolic pathways, bioRxiv: The preprint server for biology (2023).
Sheikh, N., Tron, K., Dudas, J. & Ramadori, G. Cytokine-induced neutrophil chemoattractant-1 is released by the noninjured liver in a rat acute-phase model. Lab. Investig. J. Techn. Methods Pathol. 86, 800–814 (2006).
Choong, M. L., Yong, Y. P., Tan, A. C., Luo, B. & Lodish, H. F. LIX: A chemokine with a role in hematopoietic stem cells maintenance. Cytokine 25, 239–245 (2004).
Appay, V. & Rowland-Jones, S. L. RANTES: A versatile and controversial chemokine. Trends Immunol. 22, 83–87 (2001).
Lu, J., Liu, J. & Li, A. Roles of neutrophil reactive oxygen species (ROS) generation in organ function impairment in sepsis. J. Zhejiang Univ. Sci. B 23, 437–450 (2022).
Jachova, J. et al. Neuroprotection mediated by remote preconditioning is associated with a decrease in systemic oxidative stress and changes in brain and blood glutamate concentration. Neurochem. Int. 129, 104461 (2019).
Kim, T. K. et al. Effect of remote ischaemic conditioning on coagulation function as measured by whole blood impedance aggregometry and rotational thromboelastometry in off-pump coronary artery bypass surgery: A randomised controlled trial. Thromb. Res. 187, 72–78 (2020).
Gorog, D. A. et al. Effect of remote ischaemic conditioning on platelet reactivity and endogenous fibrinolysis in ST-elevation myocardial infarction: A substudy of the CONDI-2/ERIC-PPCI randomized controlled trial. Cardiovasc. Res. 117, 623–634 (2021).
Godskesen, L. E. et al. Remote ischemic conditioning in active ulcerative colitis: An explorative randomized clinical trial. Sci. Rep. 10, 9537 (2020).
Acknowledgements
The authors gratefully acknowledge the excellent technical assistance of Dana Jurusova.
Funding
This research was funded by Slovak Research and Development Agency under Contract [APVV-21-0069], Slovak Grant Agencies [VEGA 1/0723/21 and VEGA 2/0096/22] and the Operational Programme Integrated Infrastructure for the project: Strengthening of Research, Development and Innovation Capacities of Translational Biomedical Research of Human Diseases, [IMTS: 313021BZC9], co-financed by the European Regional Development Fund.” Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic.
Author information
Authors and Affiliations
Contributions
The conceptualization was designed by J.K. with P.B., which were responsible also for funding acquisition. Material preparation, data collection and analysis were performed by J.K., P.B., K.K. and M.N. The visualisation performed M.B. The first draft of the manuscript was written by P.B. and J.K. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experiments were carried out in accordance with the protocol for animal care approved by the European Communities Council Directive (2010/63/EU) with permission of the State Veterinary and Food Administration of the Slovak Republic (4451/14–221 and 4247/15–221) under the supervision of the ethical council of the Institute of Neurobiology BMC SAS. Every effort was made to minimise animal suffering and reduce the number of animals used.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Končeková, J., Kotorová, K., Némethová, M. et al. Effectiveness of remote ischaemic conditioning is not affected by hyper-inflammation in a rat model of stroke. Sci Rep 14, 20750 (2024). https://doi.org/10.1038/s41598-024-71328-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71328-z
- Springer Nature Limited