Abstract
So far, a variety of metabolite components of kiwifruit have been elucidated. However, the identification and analysis of flavonoids in different tissues of kiwifruit are rarely carried out. In this study, we performed transcriptome and metabolome analyses of roots (Gkf_R), stems (Gkf_T), leaves (Gkf_L), and fruits (Gkf_F) to provide insights into the differential accumulation and regulation mechanisms of flavonoids in kiwifruit. Results showed that a total of 301 flavonoids were identified, in four tissues with different accumulation trends, and a large proportion of flavonoids had high accumulation in Gkf_L and Gkf_R. A total of 84 genes have been identified involved in the flavonoid biosynthesis pathway, and the expression levels of five LAR, two DFR, and one HCT were significantly correlated with the accumulation of 16 flavonoids and co-localized in the flavonoid biosynthesis pathway. In addition, a total of 2362 transcription factor genes were identified, mainly MYBs, bHLHs, ERFs, bZIPs and WRKYs, among which the expression level of bHLH74, RAP2.3L/4L/10L, MYB1R1, and WRKY33 were significantly correlated with 25, 56, 43, and 24 kinds of flavonoids. Our research will enrich the metabolomic data and provide useful information for the directed genetic improvement and application in the pharmaceutical industry of kiwifruit.
Similar content being viewed by others
Introduction
Kiwifruit is native to China and has been recorded since the Spring and Autumn Periods, it is rich in vitamins, dietary fiber, lipids, amino acids, flavonoids, minerals, and other nutrient components that are considered beneficial to human health1,2,3,4. A total of 54 species and 21 varieties in the genus Actinidia have been recognized worldwide, of which 52 are native to China. According to the Compendium of Materia Medica and Herbal Supplements, the roots, stems, leaves, and fruits of kiwifruit can be used as medicine. Modern medical research also shows that kiwifruit contains a variety of physiologically active ingredients and has significant pharmacological effects5,6,7,8. For example, kiwifruit juice can effectively block the synthesis of the carcinogens nitrosamine, N-nitrosamide, N-nitromorpholine, and N-nitrosoproline9,10. The root extracts of kiwifruit have a good effect on scavenging free radicals, inhibiting lipid peroxidation, maintaining the normal structure of the plasma membrane, and avoiding cell damage11. Through metabolomics analysis study found that the most important bioactive metabolites in kiwifruit were flavonoids12,13,14. Since the development of kiwifruit globalization in the 1960s, so far, the world’s kiwifruit cultivation area has been more than five million mu, production of more than four million tons. But all of them are for the purpose of producing fresh fruit, and the production and research work for medicinal development are rarely carried out.
Flavonoids are by far the largest group of polyphenols, with an estimated number of over 8000 flavonoid metabolites. They can be divided into the following categories: flavones, biflavones, flavanones, flavanonols, chalcones, isoflavones, flavonols, flavanols, flavonoid carbonosides, anthocyanidins, and proanthocyanidins. Among them, a variety of flavonoids have anti-oxidant, anti-inflammatory, anti-tumor, liver protection, lipid-lowering, cholesterol-lowering, reducing blood lipid, protecting the nervous system, preventing cardiovascular diseases, and other effects15,16,17,18,19,20,21. As the main secondary metabolites of plants, flavonoids also play a crucial role in the growth and development of plants. Such as affecting the synthesis of the cell wall22, participating in root growth23, pollen development24, color formation25, and improving plant resistance26,27,28,29,30.
The pathways involved in flavonoid metabolites include the flavonoid biosynthesis pathway (map00941), the anthocyanin biosynthesis pathway (map00942), the isoflavone biosynthesis pathway (map00943), and the flavone and flavonol biosynthesis pathway (map00944). Among them, map00941 synthesized upstream substances from the other three pathways, including apigenin and kaempferol in the map00944 pathway, liquiritigenin and naringenin in the map00943 pathway, and pelargonidin, cyanidin, and delphinidin in the map00942 pathway, respectively. The biosynthesis of flavonoids starts with phenylalanine andis catalyzed by the phenylpropane-related enzymes phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), and 4-coumarate-CoA ligase (4CL) to form coumaroyl-CoA. Subsequently, coumaroyl-CoA is catalyzed by the series enzymes chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase (F3H), flavonoid-3′-hydroxylase (F3′H), and flavonoid-3′,5′-hydroxylase (F3′5′H), leucoanthocyanidin reductase (LAR), and dihydroflavonol 4-reductase (DFR). UDP-glucose flavonol 3-O-glucosyltransferase (UFGT), anthocyanidin synthase (ANS), and anthocyanidin reductase (ANR) to form anthocyanins and the corresponding series products chalcone, flavanone, dihydroflavonol, flavonols, leucocyanidin, anthocyanidins, etc31,32,33,34. In addition to the above key enzyme genes, a variety of key regulatory factors, such as bHLH, MYB, WRKY, bZIP, and ERF have been reported to be involved in flavonoid biosynthesis35,36,37,38.
With the development of metabolomics, more and more endogenous small molecule metabolites can be identified and quantified. So far, a variety of metabolite components of kiwifruit have been elucidated. However, the identification and analysis of flavonoid compounds in different tissues of kiwifruit are rarely carried out. In order to elucidate the composition and content differences of flavonoids in different tissues of kiwifruit and the potential regulatory mechanism of the biosynthetic pathway. The transcriptome and metabolome analyses were used to investigate gene expression and flavonoids detection in this study. Our research will enrich the metabolomic data and provide useful information for the directed genetic improvement, and application in the pharmaceutical industry of kiwifruit.
Results
Metabolomic profiling
The UPLC-ESI–MS/MS technology platform was used for qualitative and relative content analysis of flavonoids in Actinidia chinensis cv. ‘Jinyan’. A total of 301 flavonoids were identified, mainly flavonols and flavones (Table 1, Supporting Information File 1). Among them, 218, 274, 286, and 231 flavonoids were identified in Gkf_R, Gkf_T, Gkf_L, and Gkf_F, respectively. A total of 184 flavonoids were detected in all four tissues (Supporting Information File 1). In addition, 1,7-di-o-galloyl-d-sedoheptulose (cmsn001563) specifically accumulated in Gkf_R, biochanin A-7-o-glucoside (pme3400) and acacetin-7-o-galactoside (pmp000573) specifically accumulated in Gkf_T, delphinidin-3-o-sophoroside-5-o-glucoside (lmqp001553), baicalein (mws0041), isosinensetin (pmp001076) specifically accumulated in Gkf_F, and 14 kinds of flavonoids specifically accumulated in Gkf_L (Supporting Information File 2).
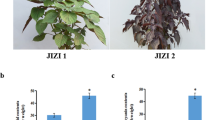
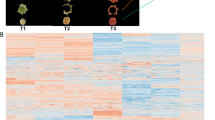
The PCA was used to elucidate the overall flavonoid differences between groups as well as the variability between samples within groups. The results showed that PC1 and PC2 could explain 48.29% and 23.48% of the total variance of the samples, indicating that there were obvious flavonoids differences in different tissues (Fig. 1c). The heatmap of hierarchical clustering analysis was used to classify the accumulation and variation model of metabolites in Gkf_R, Gkf_T, Gkf_L, and Gkf_F. The results suggested that the flavonoids in four tissues showed different accumulation trends and a large proportion of flavonoids with high accumulation in Gkf_L (Fig. 1b). Therefore, we performed further analysis of flavonoids that were differentially accumulated in different tissues.
Differentially accumulated flavonoids analysis
Univariate and multivariate statistical analyses were used to screen DAFs for each comparison group. The OPLS-DA and 200-response sorting test results showed that the model was stable and reliable (Figs. 2, S1), and the VIP analysis in the OPLS-DA model could be used to screen DAFs. A total of 279 DAFs were screened from the six comparison groups under the set threshold, and the DAFs in each comparison group are presented in Table 2. The comparison group of Gkf_L_vs_Gkf_F had the largest number of DAFs, with more than 200 flavonoids being more abundant in Gkf_L than in Gkf_F, and only 17 flavonoids being more abundant in Gkf_F. Followed by the Gkf_R_vs_Gkf_L comparison group, 157 flavonoids were up-regulated in Gkf_L, and 47 flavonoids were down-regulated in Gkf_R. The smallest difference in flavonoid content was found between Gkf_R and Gkf_T tissues, only 162 DAFs were identified in the Gkf_R_vs_Gkf_T comparison group. In addition, a total of 29 differentially accumulated flavonoids were present in all six comparison groups (Supporting Information File 3), meaning that the contents of these 29 flavonoids were significantly different in all four tissues.
Analysis of differentially accumulated flavonoids (DAFs) identified in the Gkf_L_vs_Gkf_F comparison group. Orthogonal partial least squares-discriminant analysis (OPLS-DA) (a) and 200-response sorting tests (b). Q2 is an important parameter for evaluating the OPLS-DA model, and the R2X and R2Y represent the percentage of OPLS-DA model that can explain X and Y matrix information, respectively.
K-means analysis showed that the 279 DAFs could be divided into six accumulation patterns (Fig. 3). Compared with Gkf_R, Gkf_T, and Gkf_F, a total of 121 flavonoids were highly accumulated in Gkf_L, mainly 65 flavonols, 21 flavones, eight flavanonols, seven flavanones, and six anthocyanidins. Compared with Gkf_T, Gkf_L, and Gkf_F, a total of 63 flavonoids were highly accumulated in Gkf_R, mainly 14 flavones, 10 flavonols, seven flavanols, six flavanones, six tannins, and five proanthocyanidins (Supporting Information File 4). In addition, a total of 22 and 30 flavonoids were significantly accumulated in Gkf_F and Gkf_T, respectively.
The hierarchical clustering heatmap analysis showed that in the Gkf_L_vs_Gkf_F comparison group, 38 DAFs with KEGG pathway functional annotation information were all highly accumulated in Gkf_L compared to Gkf_F (Fig. 4). In the Gkf_R_vs_Gkf_F comparison group, a total of 29 DAFs obtained KEGG pathway functional annotation, among them, eight DAFs were highly accumulated in Gkf_F, and 21 DAFs were highly accumulated in Gkf_R (Fig. S2). In the Gkf_R_vs_Gkf_T comparison group, 25 DAFs obtained KEGG pathway functional annotation, six DAFs up-regulated in Gkf_R, and 19 DAFs up-regulated in Gkf_T (Fig. S3). In the Gkf_T_vs_Gkf_F comparison group, 39 DAFs obtained KEGG pathway functional annotation, and three DAFs were up-regulated in Gkf_T and 36 DAFs were up-regulated in Gkf_F (Fig. S4). In the Gkf_T_vs_Gkf_L and Gkf_R_vs_Gkf_L comparison groups, most DAFs with KEGG pathway functional annotations were highly accumulated in Gkf_L (Fig. S5, S6). These results indicate that in ‘Jinyan’, the most abundant flavonoid components and contents are found in Gkf_L, followed by Gkf_R, Gkf_F, and Gkf_T.
Transcriptomic profiling
A total of 12 cDNA libraries for Gkf_R, Gkf_T, Gkf_L, and Gkf_F of ‘Jinyan’ were constructed and sequenced to obtain gene expression signatures at the transcription level. The average Q30 and GC content of the 12 libraries were 94.52% and 45.03%, respectively. A total of 28,783,394 clean reads were obtained and assembled into 50,439 unigenes, and 45,478 (90.16%) unigenes were annotated (Supporting Information File 5). Among them, 45,284 (99.57%), 45,120 (99.21%), 21,267 (46.76%), 27,639 (60.77%), and 35,980 (79.12%) unigenes were annotated in the Nr, UniProt, KEGG, KOG, and GO databases, respectively. Among them, a total of 84 genes have been identified involved in the flavonoids biosynthesis pathway, mainly LAR, DFR, ANS, CHI, HCT, FLS, CHS, F3H, and CYP81E/75B1/73A (Supporting Information File 6). In addition, a total of 2362 transcription factor genes were identified, including 231 MYB, 188 bHLH, 133 ERF, 107 bZIP, and 112 WRKY (Supporting Information File 7).
Differentially expressed genes analysis
Differential expression analysis showed that a total of 20,542 DEGs were identified in six comparison groups, with the threshold values. A large proportion of DEGs were obtained from the Gkf_R_vs_Gkf_L and Gkf_R_vs_Gkf_F comparison groups, and only 6351 DEGs and 5371 DEGs were identified in the Gkf_R_vs_Gkf_T and Gkf_T_vs_Gkf_L comparison groups, respectively (Table 2). The flavonoid biosynthesis pathway genes FLS, HCT, and CYP73A were highly expressed in Gkf_T, F3H, ANS, DFR, and CHS were highly expressed in Gkf_R and Gkf_T, the LAR was highly expressed in Gkf_L, and CYP81E were highly expressed in Gkf_F and Gkf_L (Supporting Information File 6). In addition, a total of 97 transcription factor genes previously reported to be involved in the regulation of flavonoid biosynthesis were significantly differentially expressed in four tissues of ‘Jinyan’. Among them, 11 MYB, six bHLH, two ERF, and one WRKY genes were significantly highly expressed in Gkf_F, 12 WRKY, 10 ERF, nine MYB, and one bHLH gene were significantly highly expressed in Gkf_L, and 21 ERF, eight MYB, seven bZIP, six bHLH, and three WRKY genes were significantly highly expressed in Gkf_R (Supporting Information File 8). To further verify the reliability of candidate gene expression levels and transcriptome data, 12 genes involved in flavonoid biosynthesis were selected for qRT-PCR analysis. The results showed that the expression characteristics of 12 genes in four tissues of ‘Jinyan’ were consistent with the results of transcriptome sequencing (Fig. 5).
To further clarify the intrinsic mechanisms underlying the differential accumulation of flavonoids in different tissues of kiwifruit, we performed an integrated analysis of transcriptome and metabolome data. The results showed that a total of 18,510 DEGs were significantly correlated with 279 DAFs (Fig. 6, Supporting Information File 9), and the number of positively correlated DEGs was significantly higher than that of negatively correlated DEGs (Figs. 7a, S7). The CCA analysis also demonstrated a high correlation between transcriptome and metabolome data (Figs. 7b, S8). However, KGML analysis showed that the vast majority of DEGs were located in the glyoxylate and dicarboxylate metabolism pathways (Ko00630), purine metabolism pathways (Ko00230), glycolysis/gluconeogenesis biosynthesis pathways (Ko00010), glycine, serine and threonine metabolism pathways (Ko00260), and pentose phosphate pathways (Ko00030) (Figs. 7c, S9). and only nine DEGs (five LAR, two DFR, two HCT) and 16 DAFs were co-located in the flavonoid biosynthesis pathways (Ko00941). Among them, the expression of HCT genes was significantly positively correlated with the accumulation level of epiafzelechin (mws1422), gallocatechin (mws0049), and kaempferol (mws1068), the expression of DFR genes was significantly negatively correlated with the accumulation level of fustin (mws1000), and the expression of LAR genes was significantly positively correlated with the accumulation level of the other 15 DAFs except for mws1000 (Supporting Information File 10, Fig. 7d).
The hierarchical clustering heatmap analysis of correlation between differentially expressed genes (DEGs) and differentially accumulated flavonoids (DAFs), each row represents a DEG, each column represents a DAF, red indicates a positive correlation between DEGs and DAFs, green represents a negative correlation.
Integrated analysis of transcriptome and metabolome data. (a) The ninequadrants analysis of differentially expressed genes (DEGs) and differentially accumulated flavonoids (DAFs) in Gkf_L_vs_Gkf_F comparison group. (b) The canonical correlation analysis of DEGs and DAFs in Gkf_L_vs_Gkf_F, purple and red dots represent DEGs and DAFs, respectively, and within the same region, further away from the origin indicates stronger correlation. (c) The KGML analysis of DEGs and DAFs in Gkf_L_vs_Gkf_F, squares represent DEGs, rhombus represent DAFs, red indicates the DEGs or DAFs up-regulation, and green indicates down-regulation, the metabolic pathway information used in the analysis was obtained from the KEGG databases (Kanehisa labs)54. (d) The correlation network analysis, red circles represent DEGs, squares represent DAFs, solid lines indicates significant positive correlations, and dashed lines represent significant negative correlations.
In addition, the specific high expression of the bHLH74 gene in fruits was significantly correlated with the accumulation levels of 25 kinds of flavonoids, the specific high expression of the ERF transcription factor genes RAP2.3L/4L/10L in roots was significantly correlated with the accumulation levels of 56 kinds of flavonoids, the specific high expression of the MYB1R1 gene in leaves was significantly correlated with the accumulation levels of 43 kinds of flavonoids, the specific high expression of the WRKY33 gene in leaves and stems was significantly correlated with the accumulation levels of 24 flavonoids (Supporting Information File 11). Further correlation analysis showed that the expression levels of bHLH74 and DRF, MYB1R1 and HCT, WRKY33, and LAR were significantly positively correlated, respectively.
Discussion
Flavonoids not only play an important role in the growth, development, flowering, fruiting, antibacterial, and disease prevention of plants but also have important medicinal value39. Actinidia species is rich in bioactive flavonoids, but most of the related studies have focused on Actinidia arguta14,40. The flavonoids composition, tissue distribution characteristics, and potential mechanism of regulation of flavonoid biosynthesis in Actinidia chinensis remain unclear. In this study, we conducted a metabolomic analysis on the Gkf_R, Gkf_T, Gkf_L, and Gkf_F of ‘Jinyan’. The results showed that a total of 301 flavonoids were identified, the number of flavonoids identified was much higher than that reported in Actinidia arguta (118 flavonoids)41 and less than that reported in Actinidia chinensis cv. ‘Donghong’ (380 flavonoids)12. This may be due to the fact that our test samples simultaneously contained roots, stems, leaves, and fruits. Another reason may be due to the differences in kiwifruit species. In addition, we detected the flavonoids in kiwifruit stems for the first time, and a total of 274 flavonoids were detected, which enriched the kiwifruit flavonoid database and provided a theoretical basis for further processing and utilization of kiwifruit pruning branches. The results of differential analysis showed that the composition and content of flavonoids were also significantly different in different tissues of ‘Jinyan’. 279 of the 301 flavonoids were differentially accumulated in two or more tissues. A total of 121 and 63 flavonoid compounds had significantly high accumulation in Gkf_L and Gkf_R, respectively. This may be related to the fact that roots and leaves are the most important and direct plant tissues in response to external environmental changes. For example, naringenin, sakuranin, and robinetin, which have been reported to be involved in drought stress, are highly accumylated in roots42. The 6,7,8-tetrahydroxy-5-methoxyflavone, 5,6,7,3′,4′-pentamethoxyflavone, tricetin, 4′,5,6,7-pentamethoxyflavone with ultraviolet absorption function are highly accumulated in leaves43. The cyanidin-3,5-O-diglucoside, quercetin-3-O-glucoside, kaempferol-3-O-glucoside, pelargonidin-3-O-rutinoside, which are associated with color formation are highly accumulated in fruits44. It also shows that the root and leaves of ‘Jinyan’ have potential value for pharmaceutical development and utilization.
Flavonoid biosynthesis in plants is mainly regulated by two types of genes45. The first are structural genes encoding enzymes that can catalyze the biosynthesis of different flavonoids. The second is regulatory genes that regulate the transcription of structural genes46. In this study, we performed transcriptome sequencing analysis to determine 84 genes encoding enzymes known to be involved in flavonoid biosynthesis: LAR, DFR, ANS, CHI, FLS, CHS, F3H, CYP81E, CYP75B1, and CYP73A. However, C4H, 4CL, ANR, UFGT, and UGT were not present in the flavonoids biosynthesis pathways of the involved ‘Jinyan’, probably due to interspecies differences39. In many plants, naringin, naringenin, saonol, kaempferol, myricetin, dihydromyricetin, dihydroquercetin, pelargonidin are catalyzed by positively regulating the expression of CHS, CHI, F3H, DFR, and FLS6. This is basically consistent with the results of the present study.
Previous studies have shown that MYB, bHLH, WD40, and their complexes (MBW) are the major regulatory genes regulating flavonoid biosynthesis47,48,49. In the present study, the largest number of transcription factors identified was MYB followed by bHLH. Notably, no WD40 TFs were identified in the ‘Jinyan’, suggesting that MYB and bHLH are the main TF families regulating flavonoid biosynthesis in ‘Jinyan’39. This is consistent with previous reports by Liu et al.50 that MYB and bHLH interact and thus exert functions on structural genes (AcMYBF110-AcbHLH-AcWDR). It has also been reported in apricot fruits that MYB5, bHLH and bHLH were positively correlated with ANS7 and CYP75B11, key structural genes for flavonoid biosynthesis39. In this study, it was also found that the expression of transcription factor genes bHLH74, MYB1R1, WRKY33, and RAP2.3L/4L/10L was significantly correlated with the accumulation of many flavonoids and the expression of flavonoid biosynthesis pathway genes DFR, LAR, and HCT. We speculate that these candidate transcription factors are involved in regulating flavonoid biosynthesis. In conclusion, in this study, the flavonoid components present in ‘Jinyan’ were identified by metabolomic analysis, which clarified the distribution characteristics of each flavonoid component in Gkf_R, Gkf_T, Gkf_L and fruit. The flavonoids related genes were identified by transcriptomic analysis, and elucidated the expression characteristics in Gkf_R, Gkf_T, Gkf_L and Gkf_F of ‘Jinyan’. Our results provide new insights into the development and utilization, and the regulation of flavonoid in ‘Jinyan’.
Materials and methods
Plant materials
The tissue samples were obtained from Fengxin County Doctor Kiwifruit Base (E114° 45, N28° 34′), Yichun City, Jiangxi Province, China. A total of 15 5-year-old kiwifruit plants were selected, and lateral roots (Gkf_R) at a depth of 15–20 cm underground, fully lignified stems (Gkf_T), fully mature leaves (Gkf_L), and fruits (Gkf_F) were collected at the physiological maturity stage (the end of September) in 2022 (Fig. 1a). Identical tissue parts from each of the five kiwifruit plants were mixed together for a total of three biological replicates, and then immediately frozen in liquid nitrogen for metabolites and total RNA extraction, respectively.
Total RNA extraction and transcriptome sequencing analysis
Total RNA from each sample was extracted using a Plant RNA Purification Reagent (Invitrogen) according to the manufacturer’s instructions. The cDNA libraries were carried out according to the protocol reported by Li et al51. Libraries were sequenced using the Illumina Novaseq 6000 platform at the Science Corporation of Gene (Guangzhou, China). The RNA sequence data were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the study accession number PRJNA1071734 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1071734). After filtering out low-quality sequencing data, the obtained clean reads were aligned and assembled using the HISAT2 software52 and the Trinity method53, respectively. Based on sequence similarity, the assembled genes were compared with Non-redundant (Nr), Kyoto Encyclopedia of Genes and Genomes (KEGG)54, Swiss-Prot, Eukaryotic Orthologous Groups (KOG), and Gene Ontology (GO) public databases with an E-value < 10−5 to obtain functional annotation information.
Differential expression and quantitative real-time PCR analyses
The expression levels of the genes were normalized and calculated as the value of fragments per kilobase of transcripts per million mapped fragments (FPKM). Differentially expressed genes (DEGs) that satisfied the parameters with |log2FC|≥ 1 and adjusted p-value ≤ 0.05 screening was carried out using the R package DESeq. A total of 12 candidate genes were selected to investigate the expression profiles through Quantitative Real-Time PCR (qRT-PCR) analyses. Reverse transcription was performed using a PrimeScript TMRT reagent kit with a gDNA Eraser (Code No: RR047A, TaKara, Dalian, China) following the instructions of the manufacturer. The primer sequence details for the 12 candidate genes and the internal control gene AcActin are shown in Table S1. The qRT-PCR analyses were performed using the LightCycler 480 System (Roche, Basel, Switzerland) and a TB Green Premix Ex Taq II kit (Code No: RR820A, TaKara). Three biological replicates of each tissue of kiwifruit were assessed, and each qRT-PCR reaction included three technical replicates. The relative expression levels of the candidate genes were calculated using the 2−ΔΔCt method55.
Metabolite extraction and widely targeted metabolome analysis
The samples were freeze-dried and crushed using a mixer mill (MM400, Retch) with zirconia beads for 1.5 min at 30 Hz. The powder (100 mg) was weighed and submerged in 1.2 ml of 70% aqueous methanol, vortex 30 s every 30 min for 6 times in total, place the sample in a refrigerator at 4℃ overnight. Following centrifugation at 12,000 rpm for 10 min, the supernatant was absorbed and filtrated (0.22-µm pore size) for ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analyses by Wuhan Metware Biotechnology Co., Ltd., China56.
The sample extracts were analyzed based on the UPLC-ESI–MS/MS (electrospray ionization, ESI) system. The analytical conditions were conducted as previously described, UPLC column, Agilent SB-C18 (1.8 µm, 2.1 × 100 mm). The mobile phase consisted of solvent A, which was pure water with 0.1% formic acid, and solvent B was acetonitrile with 0.1% formic acid. The gradient program was 95% A and 5% B at 0 min, a linear gradient to 5% A and 95% B within 9 min, and 5% A and 95% B for 1 min, and 95% A and 5% B adjusted within 0.1 min and maintained for 2.9 min. The flow rate was 0.35 mL/min with a column temperature of 40 °C, and an injection volume of 4 µl. The MS/MS conditions used an electrospray ionization temperature of 550 °C, ion spray voltages of 5500 V (positive ion mode) and − 4500 V (negative ion mode), ion source gas I (50 psi), gas II (60 psi), and curtain gas (25 psi), and the collision-activated dissociation was set to high. Each ion pair was scanned based on the optimized collision energy and declustering potential in the triple quadrupole (QQQ)56.
Qualitative and quantitative and differentially accumulated flavonoids analysis
Qualitative and quantitative analyses of the metabolites were performed using secondary spectral information based on the public metabolite database and the self-built MWBD database (Wuhan Metware Biotechnology Co., Ltd., China). The characteristic ions of each substance were screened out by the multiple reaction monitoring of QQQ57, and the signal strengths of the characteristic ions were obtained in the detector. The Analyst 1.6.3 software was used to process the mass spectrometry data. The MultiaQuant software was used to calculate the relative content of the corresponding substances in the peak area of each chromatographic peak. The multivariate analysis of variable importance in projection (VIP) in the orthogonal partial least squares-discriminant analysis (OPLS-DA) model was used to initially screen differentially accumulated flavonoids (DAFs). The DAFs were identified based on a VIP ≥ 1 and |log2FC|≥ 1. A permutation test with 200 permutations was performed to avoid overfitting.
Statistical analysis
The multivariate principal component analysis (PCA) and OPLS-DA were conducted using the base package and “MetaboAnalystR” in the R58. The K-means analysis and hierarchical cluster analysis were carried out by R package ComplexHeatmap. Pearson correlation coefficient (PCC) analysis of transcriptome and metabolome data was performed in the R. The network of genes and metabolites with a PCC > 0.8 was visualized with the software Cytoscape59. A canonical correlation analysis (CCA) was performed for genes and metabolites in the correlation network using the CCA package in the R language60. The nine-quadrant graph was used to show the DEGs and DAFs in each group with a PCC > 0.8 among different groups using “ggplot2” and “getopt” in R. The KGML analysis was based on the KEGG pathway database (http://www.kegg.jp/kegg/pathway.html).
Conclusion
In this study, we analyzed the flavonoid components and relative contents in the roots, stems, leaves and fruits of 'Jinyan' kiwifruit, and the results showed that the flavonoid components and accumulation levels in different tissues were significantly different. Flavonoid components and contents in roots and leaves were higher than those in stems and fruits. Transcriptome sequencing analysis identified 84 structural genes involved in flavonoid biosynthesis pathways, and 2036 transcription factor genes, of which 97 transcription factor genes were previously reported to be involved in regulating flavonoid biosynthesis. Further correlation and qRT-PCR analysis indicated that DFR, HCT, LAR, bHLH74, MYB1R1, WRKY33, and RAP2.3L/4L/10L may be the key genes regulating flavonoid biosynthesis in 'Jinyan' kiwifruit. Of course, further molecular function verification experiments are needed to support these conclusions.
Data availability
The RNA sequence data were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the study accession number PRJNA1071734 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1071734). All data generated or analyzed in this work are included in this published article and its supporting information files. Any other data used and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
Hu, H., Li, P., Wang, Y. & Gu, R. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 156, 100–109 (2014).
Wang, R. C. et al. Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis). New Phytol. 233, 373–389 (2022).
McGhie, T. K. Secondary metabolite components of kiwifruit. Adv. Food Nutr. Res. 68, 101–124 (2013).
Xiong, Y. et al. Nutritional component analyses of kiwifruit in different development stages by metabolomic and transcriptomic approaches. J. Sci. Food Agric. 100, 2399–2409 (2020).
Wang, X. J., Miao, L. P., Wu, T. & Shen, L. H. Advances in studies on chemical constituents in roots of Actinidia chinensis and their pharmacological activities. Chin. Tradit. Her. Dru. 42, 1233–1240 (2012).
Wang, M. X., Wang, T. Y. & Bai, N. S. Research progress in chemical components and anticancer pharmacological effects of root of Actinidia arguta planch. ex Miq. Chin. J. Inf. Tradit. Chin. Med. 26, 137–140 (2019).
Lin, Y. H., Wu, J. Z. & Wu, J. G. Studies on the purification process of triterpenoid from the root of Actinidia eriantha Benth. Strait Pharm. J. 29, 29–32 (2017).
Górniak, I., Bartoszewski, R. & Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 18, 241–272 (2019).
Li, L. The root extracts of Actinidia chinensis var. Deliciosa on the effects of carbon tetrachloride to slow liver injury of mice. Lishizhen Med. Mater. Med. Res. 22, 1448–1449 (2011).
Zhang, L. & Song, P. J. The cancer prevention of Actinidia chinensis. ACTA Nutrimenta Sinica 9, 311–316 (1987).
Bai, X. P. & Qiu, A. B. Experimental study on the liver-protecting effect of the root extracts of Actinidia chinensis var. Deliciosa. J. Chin. Med. Mater. 29, 824–827 (2006).
Zhang, L., Tang, Z., Zheng, H., Zhong, C. & Zhang, Q. Comprehensive analysis of metabolome and transcriptome in fruits and roots of kiwifruit. Int. J. Mol. Sci. 24, 1299 (2023).
Wang, Y. B. et al. Metabolomic and transcriptomic analysis of flavonoid biosynthesis in two main cultivars of Actinidia arguta Sieb.Zucc. grown in Northern China. Front. Plant Sci. 13, 911203 (2022).
Li, Y. K. et al. Combined analysis of the fruit metabolome and transcriptome reveals candidate genes involved in flavonoid biosynthesis in Actinidia arguta. Int. J. Mol. Sci. 19, 1471–1487 (2018).
Latocha, P., Wolosiak, R., Worobiej, E. & Krupa, T. Clonal differences in antioxidant activity and bioactive constituents of hardy kiwifruit (Actinidia arguta) and its year-to-year variability. J. Sci. Food Agric. 93, 1412–1419 (2013).
Jiang, L. L. et al. Bioactive compounds from plant-based functional foods: A promising choice for the prevention and management of hyperuricemia. Foods 9, 973 (2020).
Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 143, 383–396 (2012).
Devi, K. P. et al. Kaempferol and inflammation from chemistry to medicine. Pharmacol. Res. 99, 1–10 (2015).
Wang, S. Y., Bowman, L. & Ding, M. Methyl jasmonate enhances antioxidant activity and flavonoid content in blackberries (Rubus sp.) and promotes antiproliferation of human cancer cells. Food Chem. 107, 1261–1269 (2008).
Perez-Vizcaino, F. & Duarte, J. Flavonols and cardiovascular disease. Mol. Aspects. Med. 31, 478–494 (2010).
Valentova, K., Vrba, J., Bancirova, M., Ulrichova, J. & Kren, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 68, 267–282 (2014).
Saffer, A. M. & Irish, V. F. Flavonol rhamnosylation indirectly modifies the cell wall defects of rhamnose biosynthesis 1 mutants by altering rhamnose flux. Plant J. 94, 4 (2018).
Tohge, T. & Fernie, A. R. Specialized metabolites of the flavonol class mediate root phototropism and growth. Mol. Plant 9, 1554–1555 (2016).
Lan, X., Jia, Y. & Kumar, A. Flavonoids and ROS play opposing roles in mediating pollination in ornamental kale (Brassica oleracea var. acephala). Molecular Plant 10, 1361–1364 (2017).
Cao, X. et al. A putative R3 MYB repressor is the candidate gene underlying atroviolacium, a locus for anthocyanin pigmentation in tomato fruit. Plant Biotechnol. J. 68, 5745–5758 (2017).
Li, P. et al. The arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 89, 85–103 (2016).
Peng, M. et al. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nat. Commun. 8, 1975 (2017).
Murata, K. et al. Natural variation in the expression and catalytic activity of a naringenin 7-O-methyltransferase influences antifungal defenses in diverse rice cultivars. Plant J. 101, 1103–1117 (2020).
Dai, Z., Tan, J. & Zhou, C. The osmiR396-osGRF8-osF3H-flavonoid pathway mediates Resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol. J. 17, 1657–1669 (2019).
Zhang, P. P. et al. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soybean isoflavone content and resistance to soybean mosaic virus. Plant Biotechnol. J. 18, 1384–1395 (2020).
Tohge, T., de Souza, L. P. & Fernie, A. R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 68, 4013–4028 (2017).
Yonekura-Sakakibara, K., Higashi, Y. & Nakabayashi, R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 10, 943 (2019).
Shen, N. et al. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 383, 132531 (2022).
Liu, W. et al. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 22, 1282 (2021).
Ding, M. et al. FtBPM3 modulates the orchestration of FtMYB11-mediated flavonoids biosynthesis in Tartary buckwheat. Plant Biotechnol. J. 19, 1285–1287 (2021).
Yao, P. et al. FtMYB6, a light-induced SG7 R2R3-MYB transcription factor, promotes flavonol biosynthesis in tartary buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 68, 13685–13696 (2020).
Tao, R. Y. et al. Light-induced basic/helix-loop-helix 64 enhances anthocyanin biosynthesis and undergoes constitutively photomorphogenic 1-mediated degradation in pear. Plant Physiol. 184, 01188 (2020).
Chen, L. et al. Genome-wide identification of WD40 transcription factors and their regulation of the MYB-bHLH-WD40 (MBW) complex related to anthocyanin synthesis in Qingke (Hordeum vulgare L. var. nudum Hook. F.). BMC Genomics 24, 166 (2023).
Chen, Y. L. et al. Metabolomic and transcriptomice analyses of flavonoid biosynthesis in apricot fruits. Front. Plant Sci. 14, 1210309 (2023).
Tan, C. H. et al. Transcriptomic and metabolomics of flavonoid compounds in Actinidia arguta var. Arguta. J. King Saud Univ. Sci. 33, 101605 (2021).
Wang, Y. et al. Metabolomic and transcriptomic analysis of flavonoid biosynthesis in two main cultivars of Actinidia arguta Sieb.Zucc. grown in northern china. Front. Plant Sci. 13, 911203 (2022).
Gai, Z. et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plant under drought stress. Sci. Rep. 10, 12275 (2020).
Cuadra, P., Harborne, J. B. & Waterman, P. G. Increases in surface flavonoids and photosynthetic pigments in Gnaphalium luteoalbum in response to UV-B radiation. Phytohemistry 45, 1377–1383 (1997).
Griesser, M. et al. Redirection of flavonoid biosynthesis through the down-regulation of an anthocyanidin glucosyltransferase in ripening strawberry fruit. Plant Physiol. 146, 1528–1539 (2008).
Chen, W. K. et al. Dissecting the variations of ripening progression and flavonoid metabolism in grape berries grown under double cropping system. Front. Plant Sci. 8, 1912 (2017).
Liu, X. et al. Systematic analysis of O-methyltransferase gene family and identification of potential members involved in the formation of O-methylated flavonoids in Citrus. Gene 575, 458–472 (2016).
Li, J., Han, G., Sun, C. & Sui, N. Research advances of MYB transcription factors in plant Stress resistance and breeding. Plant Signal Behav. 14, 1613131 (2019).
Meng, X. et al. Comparative metabolomic and transcriptome analysis reveal distinct flavonoid biosynthesis regulation between petals of white and purple Phalaenopsis amabilis. J. Plant Growth Regul. 39, 823–840 (2019).
Hichri, I. et al. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 62, 2465–2483 (2011).
Liu, Y. et al. Transcriptional regulation of anthocyanin synthesis by MYB-bHLH-WDR complexes in kiwifruit (Actinidia chinensis). J. Agric. Food Chem. 69, 3677–3691 (2021).
Li, Y. K. et al. A key structural gene, AaLDOX, is involved in anthocyanin biosynthesis in all red-fleshed kiwifruit (Actinidia arguta) based on transcriptome analysis. Gene 648, 31–41 (2018).
Kim, D., Langmead, B. & Salzberg, S. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Haas, B. J. et al. novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Prot. 8, 1494–1512 (2013).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, 587–592 (2023).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCt method. Methods 25, 402–408 (2001).
Mao, J. P. et al. Widely targeted metabolomic, transcriptomic, and spatial metabolomics analysis reveals the potential mechanism of coloration and fruit quality formation in Actinidia chinensis cv. Hongyang. Foods 13, 233 (2024).
Wei, C. et al. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 6, 1769–1780 (2013).
Chong, J. & Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 34, 4313–4314 (2018).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interactionnetworks. Genome Res. 13, 2498–2504 (2003).
González, I., Déjean, S., Martin, P. G. P. & Baccini, A. CCA: An R package to extend canonical correlation analysis. J. Stat. Softw. 23, 1–14 (2008).
Acknowledgements
This Research was Funded by the Doctoral Projects of Jiangxi Academy of Sciences (Grant No: 2022YYB01), Talent program of Jiangxi Academy of Sciences (Grant No: 2022YRCC001), Key Research and development Project of Jiangxi Academy of Sciences (Grant No: 2023YSBG21003).
Author information
Authors and Affiliations
Contributions
J.M. and Z.G. Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing—original draf; Z.G: Data curation, Formal analysis, Investigation, Methodology; X.W: Conceptualization, Supervision, Validation, Writing—review and editing; D.Y, M.L, and L.C: Formal analysis, Data curation, Validation. All authors have reviewed and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mao, J., Gao, Z., Wang, X. et al. Integrated transcriptome and targeted metabolome analyses provide insights into flavonoid biosynthesis in kiwifruit (Actinidia chinensis). Sci Rep 14, 19417 (2024). https://doi.org/10.1038/s41598-024-70600-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70600-6
- Springer Nature Limited