Abstract
We aimed to evaluate factors associated with changes in skeletal muscle mass in hepatitis C virus (HCV)-infected patients after treatment with direct-acting antivirals (DAAs). Consecutive HCV-infected patients after treatment with DAA were recruited into the study. Patients who achieved sustained virological response (SVR); and had complete clinical information, preserved serum samples at baseline and SVR48, and skeletal muscle mass evaluations based on the psoas muscle mass index (PMI) on computed tomography at baseline and ≥ 12 months were included. Altogether, 70.7% of patients (41/58) showed increased PMI after DAA therapy, and mean relative PMI was significantly higher after DAA therapy than at baseline. There were no significant associations between baseline clinical factors routinely examined in clinical practice and increased PMI. Among factors reported to be associated with skeletal muscle loss in patients with chronic liver disease, serum zinc levels and total and free carnitine levels increased significantly after DAA therapy and only changes in serum free carnitine levels were significantly associated with an increased PMI (r = 0305, P = 0.020). In conclusion, increased skeletal muscle mass after successful HCV eradication by DAAs was significantly associated with increased serum-free carnitine levels. l-carnitine supplementation may be beneficial in patients with low skeletal muscle mass after DAA.
Similar content being viewed by others
Introduction
Hepatitis C virus (HCV) infection is a major cause of hepatocellular carcinoma (HCC) and liver transplantation1. Recently developed direct-acting antiviral (DAA) agents have been reported to result in a sustained viral response (SVR) rate of greater than 90% in patients with HCV infection, including those with decompensated liver cirrhosis, hemodialysis, and advanced age2,3,4,5. Successful HCV eradication by DAAs could decrease HCC occurrence6 and liver fibrosis7,8 and restore liver function3. Additionally, extrahepatic dysfunction, including cryoglobulinemia and abnormal lipid profiles9, could be restored after successful HCV eradication by DAAs. Regarding extrahepatic dysfunction, it has been reported that successful HCV eradication by DAAs could restore skeletal muscle mass loss10,11.
Primary sarcopenia is defined as muscle atrophy and age-related decrease in muscle strength12. Chronic liver disease, including liver cirrhosis (LC) and HCC, is one of the major causes of secondary sarcopenia12,13,14,15,16. Chronic liver disease with complicating sarcopenia has poor prognosis17. Thus, preventing and managing this loss of skeletal muscle mass in patients with chronic liver disease are clinically important.
There is evidence that skeletal muscle mass significantly increased or skeletal muscle loss could be suppressed after successful HCV eradication. However, some patients with low skeletal muscle mass have not experienced increases in skeletal muscle, even after successful HCV eradication Thus, clarifying the mechanisms underlying changes in skeletal mass has important clinical implications. However, predictive factors and molecular mechanisms underlying this increase in skeletal muscle mass after successful HCV eradication have not been determined yet10,11. In this study, we evaluated factors associated with increased skeletal muscle mass after successful HCV eradication by DAAs.
Results
Patients
HCV-infected patients receiving interferon (IFN)-free DAA regimen between October 2014 and November 2019 at Hokkaido University Hospital were screened. Out of these, 58 patients met the inclusion criteria and were included in the study.
The baseline characteristics of patients with HCV infection are shown in Table 1. The median age of patients was 74 years (range 43–90 years), and 48.2% of them (28/58) were men. The most common HCV genotype was genotype 1 (81.0%, 47/58). The number of patients treated with daclatasvir/asunaprevir, sofosbuvir/ledipasvir, sofosbuvir/ribavirin, ombitasvir/paritaprevir/ritonavir, or other agents was 13, 19, 9, 6, and 11, respectively. A total of 44.8% (26/58) had LC, and the median fibrosis (FIB)-4 index was 5.1 (range 0.7–12.0). The baseline median PMI value was 4.22 cm2/m2 (range 2.08–8.46) in all patients: 5.30 cm2/m2 (range 2.73–8.46) in men, and 3.89 cm2/m2 (range 2.08–6.50) in women.
Changes in skeletal muscle mass and related factors after successful HCV eradication by DAAs
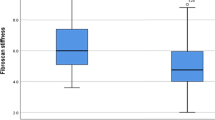
As shown in Table 1, 70.7% of patients (41/58) experienced increased PMI after DAA therapy. In addition, the relative PMI values were significantly higher after DAA treatment than those at baseline (P = 0.0056) (Fig. 1). Table 1 shows a comparison of patients with or without increased PMI after successful HCV eradication by DAAs. No factors that were routinely evaluated in the clinical practice setting differed significantly between patients with or without increased PMI after successful HCV eradication by DAAs.
Relative changes in skeletal muscle mass after successful HCV eradication by direct-acting antiviral agents (DAAs) at SVR48. SVR48; sustained virological response at 48 weeks after treatment completion. PMI psoas muscle mass index. Data are shown as means ± standard deviation (SD). Asterisks indicate a statistically significant difference (**P < 0.01).
We further analyzed factors associated with changes in skeletal muscle mass in patients with chronic liver disease. We analyzed serum vitamin D; insulin-like growth factor 1 (IGF-1); zinc, branched chain amino acid (BCAA); and total and free carnitine levels. These parameters were previously reported to be associated with skeletal muscle loss in patients with chronic liver disease18,19, at baseline and SVR48.
As shown in Fig. 2, levels of zinc (P < 0.0001), total carnitine (P < 0.0001), and free carnitine (P = 0.0051) were significantly higher after successful HCV eradication than those at baseline. Serum 25-hydroxyvitamin D (25(OH)-vitamin D), Insulin-like growth factor 1(IGF-1), and BCAA levels did not change after DAA therapy. Subsequently, we compared the levels at baseline and at the time that SVR was achieved between patients with or without PMI after successful HCV eradication. As shown in Fig. 3a,b, the levels of 25(OH)-vitamin D, IGF-1, BCAA, zinc, total carnitine, and free carnitine were similar between patients with or without PMI increase at baseline and SVR48.
Comparison of serum vitamin D, IGF-1, zinc, BCAA, and total and free carnitine levels between baseline and at SVR48. SVR48; sustained virological response at 48 weeks after the completion of treatment. IGF1 insulin-like growth factor-1, BCAA branched chain amino acid, 25-OH vitamin D 25-hydroxyvitamin D, PMI psoas muscle mass index. Data are shown as mean ± standard deviation (SD). Asterisks indicate statistically significant differences (**P < 0.01, ****P < 0.0001).
Comparison of serum vitamin D, IGF-1, zinc, BCAA, and total and free carnitine levels at baseline or SVR 48 between patients with and without PMI increases. (a) Comparison of serum vitamin D, IGF-1, zinc, BCAA, and total and free carnitine levels at baseline between patients with and without a PMI increase. (b) Comparison of serum vitamin D, IGF-1, zinc, BCAA, and total and free carnitine levels at SVR48 between patients with and without a PMI increase. SVR48 sustained virological response at 48 weeks after the completion of treatment. IGF1 insulin-like growth factor-1, BCAA branch chain amino acid, 25-OH vitamin D 25-hydroxyvitamin D, PMI psoas muscle mass index. Data are shown as mean ± standard deviation (SD). Asterisk indicate statistically significant differences (**P < 0.01, ****P < 0.0001).
Subsequently, we analyzed correlations between ΔPMI/month and serum total/free creatinine, vitamin D, IGF-1, BCAA, and zinc levels. As shown in Fig. 4, only changes in free carnitine were significantly correlated with ΔPMI/month (r = 0.305, P = 0.025).
Correlations between changes in the psoas muscle mass index (PMI)/month (ΔPMI/month) and changes in serum total/free creatinine, vitamin D, IGF-1, BCAA, and zinc. IGF1 insulin-like growth factor-1, BCAA branch chain amino acid, 25(OH)-vitamin D 25-hydroxyvitamin D, PMI psoas muscle mass index. Data were analyzed using Spearman’s rank correlation.
Thus, increased free carnitine levels were clearly associated with an increased PMI after successful HCV eradication. Among patients with increased free carnitine levels after DAA therapy, three patients were administered l-carnitine (two for muscle cramps and one for hyperammonemia) during the observational period. Accordingly, we stratified patients according to l-carnitine administration. As shown in supplementary Table S1, the baseline patients’ characteristics were similar between patients with or without L-carnitine administration. As shown in Fig. 5a, even in patients without carnitine initiation during the observational period, the relative PMI values increased significantly (Fig. 5a) and serum total and free carnitine levels were significantly higher after successful HCV eradication by DAAs than at baseline (Fig. 5b). In addition, ΔPMI/month was significantly positively correlated with changes in free carnitine levels (Fig. 5c, r = 0.302, P = 0.025) in patients without l-carnitine administration.
Comparison of data between patients with and without l-carnitine supplementation within the observational period. (a) Relative changes in the psoas muscle mass index (PMI) at SVR48 in patients with and without l-carnitine supplementation. (b) Changes in serum total and free carnitine levels between baseline and at SVR48 in patients with and without l-carnitine initiation. (c) Correlations between psoas muscle mass index (PMI) changes/month (ΔPMI/month) and changes in serum total/free creatinine in patients without l-carnitine initiation. PMI psoas muscle mass index. Data were analyzed using Spearman’s rank correlation.
All three patients treated with carnitine during the observational period exhibited increased PMI and free carnitine levels (Fig. 5).
Discussion
Chronic liver disease is a major cause of secondary sarcopenia that is associated with poor prognosis in patients with chronic liver disease. Thus, proper management and prevention of sarcopenia are highly required in patients with chronic liver disease17. Recent reports have revealed that successful HCV eradication could increase skeletal muscle mass or suppress loss of skeletal muscle mass10,11,20. In this study, 70.7% of patients showed an increase in PMI and the mean relative PMI significantly increased after successful HCV eradication by DAAs. However, clinical factors examined in routine clinical practice, including age (supplementary Figure S1), did not show increased PMI values. Among the factors that were reported to be associated with skeletal muscle loss in patients with chronic liver disease, serum zinc as well as serum total and free carnitine levels increased significantly after DAA therapy. Additionally, only increased serum-free carnitine levels were significantly associated with increased PMI.
In patients with chronic liver disease, both suppression of skeletal muscle protein synthesis and promotion of skeletal muscle catabolism are thought to be involved in skeletal muscle mass loss. Vitamin D is involved in myoblast proliferation and differentiation21,22 and vitamin D deficiency is sometimes observed in patients with chronic liver disease. Thus, in patients with chronic liver disease, vitamin D deficiency is thought to be a cause of skeletal muscle mass loss. Similarly, IGF-1 deficiency, which is mainly produced in the liver via growth hormone stimulation, was observed in patients with chronic liver disease23,24. Because IGF-1 is associated with protein synthesis, it could result in skeletal muscle mass loss. Decreased liver function due to LC causes protein energy malnutrition; in such situations, BCAA, which has strong anabolic effects, is utilized in skeletal muscle mass25. Decreased BCAA levels cause dysfunctional protein synthesis. Furthermore, serum zinc levels were decreased in patients with chronic liver disease, and this decrease was significantly associated with sarcopenia19. Therefore, in these patients with chronic liver disease, decreased IGF-1, carnitine, BCAA, zinc, and vitamin D levels are important factors of skeletal muscle mass loss. In the present study, however, IGF-1, BCAA, and vitamin D levels did not change after successful HCV eradication, and changes in serum zinc levels were not associated with those in PMI. Thus, we suggest that increased PMI might not be associated with changes in serum IGF-1, BCAA, zinc, or vitamin D levels.
As shown in Fig. 2, serum total carnitine and free carnitine levels increased significantly after successful HCV eradication by DAAs. In addition, free carnitine alone was significantly and positively correlated with ΔPMI/month. Thus, increased serum-free carnitine level was associated with increased PMI after successful HCV eradication by DAAs. Carnitine is an essential nutrient that is mainly involved in fatty acid metabolism26. Total carnitine comprises free carnitine and all acylcarnitines27 and most of carnitine exists as free carnitine28. Blood free carnitine less than < 20 μmol/L is defined as carnitine deficiency. Although carnitine is absorbed mainly from dietary intake, approximately 25% of carnitine is generated in the liver and kidney29. Thus, in patients with chronic liver or kidney disease, secondary carnitine deficiency is usually observed26,30. The effect of carnitine deficiency could be predicted based on patients with primary carnitine deficiency, who showed hepatic steatosis, hyperammonemia, and skeletal myopathy31,32. Thus, carnitine is thought to be involved in muscle conditions. Recently, we have reported that carnitine supplementation in patients with LC could suppress skeletal muscle mass loss18. The mechanisms by which carnitine suppresses skeletal muscle loss and by which increased serum-free carnitine increases PMI have not been fully clarified. However, several hypotheses have been proposed. Carnitine exists at high concentrations in the muscle33; thus, skeletal muscle might be strongly affected by a carnitine deficiency.
Hyperammonemia was observed in patients with primary carnitine deficiency and could cause muscle atrophy via upregulation of myostatin, which suppressed muscle synthesis34. We and other studies have revealed that carnitine supplementation could restore hyperammonemia18. In addition, based on in vitro and clinical analyses, we have previously reported that carnitine supplementation could suppress inflammatory parameters and oxidative stress in liver disease18,35. Since inflammation and increased oxidative stress could cause skeletal muscle loss36, the anti-inflammatory and anti-oxidative stress effects of carnitine might contribute to increased PMI after successful HCV eradication by DAAs.
The mechanisms underlying increased carnitine levels after successful HCV eradication are not clear. However, since increased carnitine levels are significantly correlated with increased serum albumin levels (Supplementary Figure S2), restoration of liver function after successful HCV eradication might be involved. Further analyses are required to clarify this association.
Additionally, in all three patients with l-carnitine supplementation, a significant increase in PMI was observed. Beneficial effects of L-carnitine supplementation in patients with chronic liver disease, including improvement of impaired brain function37, muscle cramp38, and restoring hyperammonemia18, have been reported. This study results might highlight that in patients with low skeletal muscle mass even after DAA therapy, l-carnitine supplementation with is a potential therapeutic option.
This study has several limitations. First, this was a single-center retrospective study, and the number of patients was limited. In addition, data of several factors, including serum ammonia, were lacking owing to the retrospective nature of the study. In this study, we could not show the baseline factors predicting the increased skeletal muscle mass after successful HCV eradication. Therefore, a large multicenter prospective study is required.
In conclusion, increased skeletal muscle mass after successful HCV eradication by DAAs was significantly associated with increased serum-free carnitine levels. This finding might suggest that l-carnitine supplementation may be beneficial in patients with low skeletal muscle mass after DAA therapy.
Materials and methods
Patients and study design
HCV-infected patients who were treated with DAAs and achieved SVR between October 2014 and November 2019 at Hokkaido University Hospital were retrospectively screened. Patients were included if they had paired computed tomography (CT) examinations at baseline (within 6 months before DAA initiation) and at 12 months or longer after completion of treatment, proper clinical information, and preserved serum samples obtained at baseline and at 12 months after treatment completion (SVR48). Patients were excluded if they could not achieve SVR, did not have paired CT examinations, did not have preserved serum samples at both baseline and SVR48, had insufficient clinical data, had other liver diseases, had a history of liver transplantation or hemodialysis, or were co-infected with human immunodeficiency virus or hepatitis B virus.
Clinical data of patients, CT results of skeletal muscle mass analysis, and preserved serum samples at baseline and SVR48 were collected. In addition, concomitant drugs use was monitored during the observational period, including l-carnitine, which inhibits loss of skeletal muscle mass.
Presence of LC was diagnosed by liver biopsy, FibroScan data, radiologic findings, and laboratory data18. Preserved serum was used to analyze serum levels of 25(OH)-vitamin D, total/free carnitine, zinc, BCAA, and IGF-1, which have been reported to be associated with skeletal muscle loss in patients with liver disease18,19. Serum IGF1 and 25(OH)-vitamin D were analyzed by the immunoradiometric assay, as previously described18. Serum concentrations of total and free carnitine, zinc, and BCAA levels were analyzed at the clinical laboratory of SRL (Tokyo, Japan).
The study protocol was approved by the Ethics Committee of Hokkaido University Hospital (approval numbers: 016-0021 and 016-0397). In addition, the study protocol conformed to the ethical guidelines of the Declaration of Helsinki. All patients included in the study provided written informed consent prior to participation.
Calculation of skeletal muscle mass
Skeletal muscle mass was evaluated using the psoas muscle mass index (PMI), as previously described39,40. Briefly, the PMI was calculated from paired CT as the sum of cross-sectional areas at the L3 level of the right and left psoas muscle masses by manual tracing; this value was divided by height squared18,39,40. The PMI was evaluated at baseline and at ≥ 12 months after treatment completion.
Additionally, monthly changes in the PMI (ΔPMI/month) were evaluated as follows: (psoas muscle area on CT scan at ≥ 12 months after DAA completion – psoas muscle area on the baseline CT scan)/psoas muscle area on the baseline CT scan]/interval between CT scans (months)11,39,40.
Statistical analysis
Continuous variables were analyzed using the Mann–Whitney U test or paired t-test. Categorical variables were analyzed using Fisher’s exact test. Relationships between two variables were assessed using the Spearman’s rank correlation. All statistical analyses were performed using Prism 7.03 (GraphPad Software, Inc., La Jolla, CA, USA). All P-values were two-tailed, and P < 0.05 indicated statistical significance.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kim, W. R. et al. OPTN/SRTR 2011 annual data report: Liver. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surg. 13(Suppl 1), 73–102. https://doi.org/10.1111/ajt.12021 (2013).
Suda, G. et al. Daclatasvir and asunaprevir in hemodialysis patients with hepatitis C virus infection: A nationwide retrospective study in Japan. J. Gastroenterol. 53, 119–128. https://doi.org/10.1007/s00535-017-1353-y (2018).
Takehara, T. et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: An open-label phase 3 trial. J. Gastroenterol. 54, 87–95. https://doi.org/10.1007/s00535-018-1503-x (2019).
Suda, G., Ogawa, K., Morikawa, K. & Sakamoto, N. Treatment of hepatitis C in special populations. J. Gastroenterol. 53, 591–605. https://doi.org/10.1007/s00535-017-1427-x (2018).
Suda, G. & Sakamoto, N. Recent advances in the treatment of hepatitis C virus infection for special populations and remaining problems. J. Gastroenterol. Hepatol. https://doi.org/10.1111/jgh.15189 (2020).
Nagata, H. et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 67, 933–939. https://doi.org/10.1016/j.jhep.2017.05.028 (2017).
Kawagishi, N. et al. Baseline elevated serum angiopoietin-2 predicts long-term non-regression of liver fibrosis after direct-acting antiviral therapy for hepatitis C. Sci. Rep. 11, 9207. https://doi.org/10.1038/s41598-021-88632-7 (2021).
Kawagishi, N. et al. High serum angiopoietin-2 level predicts non-regression of liver stiffness measurement-based liver fibrosis stage after direct-acting antiviral therapy for hepatitis C. Hepatol. Res. 50, 671–681. https://doi.org/10.1111/hepr.13490 (2020).
Kawagishi, N. et al. Liver steatosis and dyslipidemia after HCV eradication by direct acting antiviral agents are synergistic risks of atherosclerosis. PLoS ONE 13, e0209615. https://doi.org/10.1371/journal.pone.0209615 (2018).
Sugimoto, R. et al. Changes in liver function and body composition by direct-acting antiviral therapy for hepatitis C virus infection. Hepatol. Res. 48, 337–344. https://doi.org/10.1111/hepr.12999 (2018).
Tokuchi, Y. et al. Changes in the estimated renal function after hepatitis C virus eradication with direct-acting antiviral agents: Impact of changes in skeletal muscle mass. J. Viral Hepat. 28, 755–763. https://doi.org/10.1111/jvh.13484 (2021).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423. https://doi.org/10.1093/ageing/afq034 (2010).
Dasarathy, S. Consilience in sarcopenia of cirrhosis. J. Cachexia Sarcopenia Muscle 3, 225–237. https://doi.org/10.1007/s13539-012-0069-3 (2012).
Hanai, T. et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 46, 743–751. https://doi.org/10.1111/hepr.12616 (2016).
Iritani, S. et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 50, 323–332. https://doi.org/10.1007/s00535-014-0964-9 (2015).
Masuda, T. et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 20, 401–407. https://doi.org/10.1002/lt.23811 (2014).
Nishikawa, H. et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 46, 951–963. https://doi.org/10.1111/hepr.12774 (2016).
Ohara, M. et al. L-carnitine suppresses loss of skeletal muscle mass in patients with liver cirrhosis. Hepatol. Commun. 2, 906–918. https://doi.org/10.1002/hep4.1207 (2018).
Nishikawa, H. et al. Serum zinc concentration and sarcopenia: A close linkage in chronic liver diseases. J. Clin. Med. 8, 2. https://doi.org/10.3390/jcm8030336 (2019).
Sakamori, R. et al. Improvement of skeletal muscle mass after ledipasvir and sofosbuvir treatment for hepatitis C virus in decompensated liver cirrhosis. Intern. Med. 60, 745–750. https://doi.org/10.2169/internalmedicine.6029-20 (2021).
Ceglia, L. et al. Multi-step immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. J. Mol. Histol. 41, 137–142. https://doi.org/10.1007/s10735-010-9270-x (2010).
Buitrago, C. G., Arango, N. S. & Boland, R. L. 1alpha,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J. Cell Biochem. 113, 1170–1181. https://doi.org/10.1002/jcb.23444 (2012).
Assy, N. et al. Growth hormone-stimulated insulin-like growth factor (IGF) I and IGF-binding protein-3 in liver cirrhosis. J. Hepatol. 27, 796–802 (1997).
Donaghy, A. J., Delhanty, P. J., Ho, K. K., Williams, R. & Baxter, R. C. Regulation of the growth hormone receptor/binding protein, insulin-like growth factor ternary complex system in human cirrhosis. J. Hepatol. 36, 751–758 (2002).
Moriwaki, H. et al. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem. Biophys. Res. Commun. 313, 405–409 (2004).
Flanagan, J. L., Simmons, P. A., Vehige, J., Willcox, M. D. & Garrett, Q. Role of carnitine in disease. Nutr. Metab. 7, 30. https://doi.org/10.1186/1743-7075-7-30 (2010).
Reuter, S. E. & Evans, A. M. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet 51, 553–572 (2012).
Gnoni, A., Longo, S., Gnoni, G. V. & Giudetti, A. M. Carnitine in human muscle bioenergetics: can carnitine supplementation improve physical exercise?. Molecules https://doi.org/10.3390/molecules25010182 (2020).
Mitwalli, A. H. et al. L-carnitine supplementation in hemodialysis patients. Saudi J. Kidney Dis. Transpl. 16, 17–22 (2005).
Daniel Rudman, C. W. S. & Joseph, D. A. Deficiency of carnitine in cachectic cirrhotic patients. J. Clin. Investig. 60, 716–723 (1977).
Koizumi, T., Nikaido, H., Hayakawa, J., Nonomura, A. & Yoneda, T. Infantile disease with microvesicular fatty infiltration of viscera spontaneously occurring in the C3H-H-2(0) strain of mouse with similarities to Reye’s syndrome. Lab. Anim. 22, 83–87 (1988).
Magoulas, P. L. & El-Hattab, A. W. Systemic primary carnitine deficiency: An overview of clinical manifestations, diagnosis, and management. Orphanet. J. Rare Dis. 7, 68. https://doi.org/10.1186/1750-1172-7-68 (2012).
Brass, E. P. Supplemental carnitine and exercise. Am. J. Clin. Nutr. 72, 618S-623S (2000).
McPherron, A. C., Lawler, A. M. & Lee, S. J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90. https://doi.org/10.1038/387083a0 (1997).
Tsukuda, Y. et al. Anti-adipogenic and antiviral effects of l-carnitine on hepatitis C virus infection. J. Med. Virol. 89, 857–866. https://doi.org/10.1002/jmv.24692 (2017).
Silverio, R., Laviano, A., Rossi Fanelli, F. & Seelaender, M. l-carnitine and cancer cachexia: Clinical and experimental aspects. J. Cachexia Sarcopenia Muscle 2, 37–44. https://doi.org/10.1007/s13539-011-0017-7 (2011).
Nakanishi, H. et al. Impaired brain function improved by L-carnitine in patients with cirrhosis: Evaluation using near-infrared spectroscopy. Sci. Rep. 10, 13566. https://doi.org/10.1038/s41598-020-70585-y (2020).
Nakanishi, H. et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 13, 1540–1543. https://doi.org/10.1016/j.cgh.2014.12.005 (2015).
Ohara, M. et al. Analysis of the optimal psoas muscle mass index cut-off values, as measured by computed tomography, for the diagnosis of loss of skeletal muscle mass in Japanese people. Hepatol. Res. 50, 715–725. https://doi.org/10.1111/hepr.13499 (2020).
Ohara, M. et al. Computed tomography, not bioelectrical impedance analysis, is the proper method for evaluating changes in skeletal muscle mass in liver disease. JCSM Rapid Commun. 3, 103–114. https://doi.org/10.1002/rco2.20 (2020).
Funding
This study was supported in part by grants from the Japan Agency for Medical Research and Development (AMED; Grant numbers JP21fk0210072, JP21fk0310101, JP21fk0210047, JP21fk0210064, JP21fk0210056, JP21fk0210048, JP21fk0210058, JP21fk0210066, and JP21fk0210067) and SPS KAKENHI (Grant number 19K08458).
Author information
Authors and Affiliations
Contributions
Y.T. and G.S. designed the study, performed the statistical analyses, and wrote the manuscript. M.K., O.M., K.S., Ma.N., T.S., R.Y., T.K., Q.F., Z.Y., A.K., S.Y., S.H., M.O., N.K., and K.O. performed data collection. Mi.N., K.M., S.O., and K.O. provided hepatological advice and edited the manuscript. N.S. revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
Professor Naoya Sakamoto received lecture fees from Bristol Myers Squibb and Pharmaceutical K. K., grants and endowments from MSD K. K and Chugai Pharmaceutical Co., Ltd., and a research grant from Gilead Sciences Inc. Dr. Goki Suda received research grants from Bristol Myers Squibb. The other authors have no conflicts of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tokuchi, Y., Suda, G., Kimura, M. et al. Possible correlation between increased serum free carnitine levels and increased skeletal muscle mass following HCV eradication by direct acting antivirals. Sci Rep 11, 16616 (2021). https://doi.org/10.1038/s41598-021-96203-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96203-z
- Springer Nature Limited