Abstract
Two-dimensional (2D) structures composed of atomically thin materials with high carrier mobility have been studied as candidates for future transistors1,2,3,4. However, owing to the unavailability of suitable high-quality dielectrics, 2D field-effect transistors (FETs) cannot attain the full theoretical potential and advantages despite their superior physical and electrical properties3,5,6. Here we demonstrate the fabrication of atomically thin single-crystalline Al2O3 (c-Al2O3) as a high-quality top-gate dielectric in 2D FETs. By using intercalative oxidation techniques, a stable, stoichiometric and atomically thin c-Al2O3 layer with a thickness of 1.25 nm is formed on the single-crystalline Al surface at room temperature. Owing to the favourable crystalline structure and well-defined interfaces, the gate leakage current, interface state density and dielectric strength of c-Al2O3 meet the International Roadmap for Devices and Systems requirements3,5,7. Through a one-step transfer process consisting of the source, drain, dielectric materials and gate, we achieve top-gate MoS2 FETs characterized by a steep subthreshold swing of 61 mV dec−1, high on/off current ratio of 108 and very small hysteresis of 10 mV. This technique and material demonstrate the possibility of producing high-quality single-crystalline oxides suitable for integration into fully scalable advanced 2D FETs, including negative capacitance transistors and spin transistors.
Similar content being viewed by others
Main
As silicon (Si) field-effect transistors (FETs) approach the fundamental limits in scaling, new generations of semiconducting channels are required to reduce the short-channel effects1,2. Two-dimensional (2D) materials such as molybdenum disulfide (MoS2), which is atomically thin and has high carrier mobility, thus have huge potential in future transistors4,8,9,10. Although 2D materials have better physical and electrical properties than Si, suitable high-quality dielectric materials are not available. Therefore, FET devices based on 2D materials cannot fulfil the full potential predicted theoretically5,6. Amorphous oxide dielectrics that work well in Si technology, for example, SiO2, Al2O3 and HfO2, cannot provide a uniform and well-defined interface with 2D materials because of the disruption of long-range orders11,12,13. Considering the amorphous nature and ill-defined interfaces, there are difficulties in eliminating charge scattering and traps, thus resulting in (1) high gate leakage current (J > 1.5 × 10−2 A cm−2), (2) high interface state density (Dit > 1010 cm−2 eV−1) and (3) low dielectric strength (Ebd < 10 MV cm−1), which cannot meet the requirements stipulated by the International Roadmap for Devices and Systems (IRDS)3,5,7. Moreover, owing to the inert dangling-bond-free surface of 2D materials, depositing an atomically thin oxide without damaging the adjacent layer remains challenging6.
Compared with amorphous oxides, crystalline dielectric materials such as hexagonal boron nitride (hBN)14, calcium fluoride (CaF2) (ref. 11) and perovskite strontium titanium oxide (SrTiO3) (ref. 13) have atomically flat surfaces that bond well for smoother dielectric/2D material interfaces. In theory, these materials can overcome the problems of interface quality and defect bands. However, there are some disadvantages of crystalline dielectrics. For example, because of the relatively narrow bandgap and low permittivity, hBN with an ultrathin physical thickness exhibits extremely high leakage currents (J > 103 A cm−2) (ref. 15). The use of CaF2 and SrTiO3 is limited to back-gate FETs11,13, although large-scale integrated circuits require top-gate 2D FETs. The unique advantage that Bi2SeO5 offers is inherently tied to its semiconducting counterpart, which means it may not provide the same benefits when paired with other 2D materials16. Other crystalline dielectrics, such as mica, have difficulty in precisely controlling the area and thickness of insulators generated by exfoliation or chemical growth methods17. Furthermore, it is challenging to attain an interface state density of 1010 cm−2 eV−1 from devices comprising crystalline dielectrics. Moreover, wafer-scale synthesis of high-quality crystalline dielectrics is quite challenging11,13,16,17. As there is still no clear strategy on how to scale dielectrics down to be atomically thin as required by commercial FETs5,6, it may be necessary to identify radically different approaches for 2D devices.
Atomically thin metal oxides have attracted attention recently because of their unique electronic, optical and magnetic properties that are rarely found in their bulk counterparts18,19. By adopting simple oxidation, a stable, stoichiometric and atomically thin oxide layer can be formed on the metal surface for 2D devices18,19. So far, oxides made of transition metals such as HfO2, TiO2, Fe2O3 and Ni2O3; post-transition metals such as Al2O3; and rare earth metals, including Gd2O3, have been proposed18,19. These atomically thin metal oxides have adequate dielectric properties and atomically flat surfaces, making them suitable for electrostatic modulation of the channels of 2D materials to overcome the present limitations.
In this study, a single-crystalline Al2O3 (c-Al2O3) is achieved as a high-quality dielectric layer on the 2D MoS2 FET. By combining epitaxial lift-off and intercalative oxidation, an atomically thin c-Al2O3 with a thickness of 1.25 nm is prepared, which is thinner than the conventional oxides used in advanced Si transistors20,21. Owing to the favourable crystalline structure and well-defined interfaces, the gate leakage current (J < 1 × 10−6 A cm−2), interface state density (Dit = 8.4 × 109 cm−2 eV−1) and dielectric strength (Ebd = 17.4 MV cm−1) of the atomically thin c-Al2O3 can meet the IRDS requirements for low-power devices3,5. By using the van der Waals (vdW) transfer method, the entire FET stack, including the source, drain, dielectric and gate, can be transferred to the MoS2 channel in a one-step process to produce a 2D FET with excellent contact and dielectric interfaces. The top-gate MoS2 FET shows a steep subthreshold swing (SS) of 61 mV dec−1, ultrahigh on/off current ratio of 108 and small hysteresis of 10 mV. Excellent processing reproducibility and uniformity are demonstrated by fabricating a batch of 100 devices.
Figure 1a shows a scalable method to synthesize high-quality atomically thin c-Al2O3 layers without complex chemistry or sophisticated equipment. A graphene (Gr)/germanium (Ge) wafer is used as the template, and wafer-scale single-crystalline Al is produced by electron beam evaporation using the vdW epitaxy approach22,23,24. Given that Gr/Ge substrates can be manufactured up to 12 inches in diameter considering the epitaxy of Ge on Si, the industrial-scale production of single-crystalline Al becomes a tangible possibility. The cross-sectional high-resolution transmission electron microscopy (HR-TEM) image and X-ray diffraction results are shown in Extended Data Fig. 1 to confirm the wafer-scale vdW epitaxy of single-crystalline Al(111) on single-crystalline Gr/Ge(110). We proved that a patterned metal with an atomically flat surface can be delaminated from graphene by exploiting the weak vdW force25. In this case, Al can be peeled off readily from graphene and then oxidized mildly at room temperature in the 0.2-ppm oxygen environment. Owing to the atomically flat single-crystalline Al surface and oxygen-deficient environment (0.2 ppm O2), homogeneous coverage of chemisorbed oxygen atoms and limited penetration of oxygen can be attained. Consequently, a depolarized c-Al2O3 layer is formed when one oxygen atom chemisorbs on an Al atom, which has been confirmed theoretically and experimentally19. In only a few seconds, an ultrathin c-Al2O3 layer with a thickness of several nanometres is formed by the layer-by-layer mechanism. The cross-sectional HR-TEM image presented in Extended Data Fig. 2 shows the epitaxial relationship between c-Al2O3 (0001) and Al (111). Finally, the atomically thin c-Al2O3 is transferred onto a target substrate such as SiO2, MoS2 or Au to fabricate the device. The fabrication details are presented in the Methods and Extended Data Fig. 3. To prevent Al from oxidizing in H2O, the entire process is water-free. Moreover, benefiting from the water-free transfer process, the graphene surface is not damaged thereby allowing repeated use of the Gr/Ge wafer (Extended Data Fig. 4).
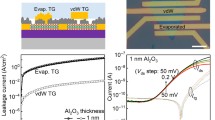
a, Fabrication of atomically thin c-Al2O3 through epitaxial lift-off and intercalative oxidation of a single-crystalline Al film. b, Optical image of the lift-off of Al/c-Al2O3 from a 4-inch Gr/Ge wafer. c, A cross-sectional HR-TEM image of Al/c-Al2O3 on a target SiO2 substrate. d, Magnified atomic-resolution image from the red box in c. e, EEL mapping of Al/c-Al2O3/SiO2. f, The relationship between exposure time in an oxygen-deficient environment (0.2 ppm O2) and the thickness of c-Al2O3. Insets, the cross-sectional HR-TEM image of c-Al2O3 with a thickness of 2 nm and 6 nm obtained by 1 h oxidation and 5 h oxidation, respectively. g, The breakdown field of c-Al2O3 and a-Al2O3. h, The breakdown field compared with film thickness for various dielectrics. Scale bars, 2 nm (c); 1 nm (d,e).
Figure 1b shows that the lift-off Al/c-Al2O3 has close to 100% yield as evidenced by delamination of the 4-inch patterned Al film from the Gr/Ge substrate using polyvinyl alcohol; Supplementary Video 1 shows the entire peeling process. The lifted-off c-Al2O3 films are transferred onto the SiO2 substrate, and the cross-sectional HR-TEM image is shown in Fig. 1c. The thickness of the c-Al2O3 layer on the Al surface is about 1.25 nm. The magnified atomic-resolution image of the red box in Fig. 1c is shown in Fig. 1d disclosing the sandwiched structure of Al/c-Al2O3/SiO2. Electron energy loss spectroscopy (EEL) mapping shows the elemental distributions (Fig. 1e), and the distributions of Al and O are in accordance with the sandwiched structure.
Moreover, the thickness of c-Al2O3 can be manipulated by exposing the vdW epitaxial Al layer to an oxygen-deficient environment for various durations. As shown in Fig. 1f, as the oxidation time is increased from 1 h to 12 h, the oxidation rate decreases from 2 nm h−1 to 0.8 nm h−1. This is because the oxidation process is controlled by the interface reaction at first and then by oxygen diffusion16. Figure 1f (insets) shows the cross-sectional HR-TEM image of c-Al2O3 with thicknesses of 2 nm (oxidation for 1 h) and 6 nm (oxidation for 5 h), and more HR-TEM images are shown in Extended Data Fig. 5. The results confirm that it is possible to prepare c-Al2O3 layers with different thicknesses by controlling the oxidation time. Moreover, the thickness mapping of the 4-inch c-Al2O3/Al wafer is shown in Supplementary Fig. 1 and Supplementary Table 1, which indicates the exceptionally uniform coefficient of variation of ±6%.
Figure 1g shows the breakdown field (Ebd) compared with the current density trend. c-Al2O3 remains completely insulating for the operating fields well above the IRDS requirement of 10 MV cm−1. The average Ebd determined from 20 metal–insulator–metal (MIM) devices with the c-Al2O3 insulator is 16 MV cm−1 and the maximum is 17.4 MV cm−1. In comparison, the amorphous Al2O3 (a-Al2O3) layer with a thickness of 5 nm fabricated by lifting off the Al film in an ambient environment exhibits a small average Ebd of about 8 MV cm−1 similar to the previous studies26,27. The enhancement of Ebd may arise from the energy gap increment or the defect density reduction in crystalline insulators5,28. The Ebd versus film thickness for different metal oxides is compared in Fig. 1h, and the details are provided in Supplementary Table 2. Owing to the crystalline structure and large bandgap (8.8 eV) (refs. 26,28), defect-assisted tunnelling in c-Al2O3 is suppressed. As a result, the atomically thin c-Al2O3 exhibits one of the highest Ebd (17.4 MV cm−1) compared with amorphous oxides (TiO2 (ref. 29), Al2O3 (ref. 30) and HfO2 (refs. 31,32,33) as well as single-crystalline oxides (CaF2 (ref. 34), SrTiO3 (ref. 13) and Sb2O3 (ref. 12)). It is important to note that Ebd of c-Al2O3 exceeds the IRDS requirement for low-power devices (Ebd > 10 MV cm−1) (refs. 3,5).
The gate leakage currents (J) and interface state densities (Dit) are important properties of low-power devices, and J and Dit should be less than 1.5 × 10−2 A cm−2 and 1010 cm−2 eV−1, respectively, to meet the IRDS requirements for low-power devices3,5,32. However, it is challenging to meet these stringent requirements for 2D devices because the amorphous oxide cannot provide a well-defined interface5,32, whereas crystalline dielectric materials are easy to form an abrupt interface with 2D channel, but some of them, for example, Sb2O3 (ref. 12), SrTiO3 (ref. 13), h-BN (ref. 15), have a relatively small bandgap. It is possible to address these problems by using single-crystalline c-Al2O3 because it has an adequate bandgap of 8.8 eV compared with other high-κ oxides26. To determine the Al/c-Al2O3 gate properties, the structure is transferred onto the multilayer MoS2 to form the Al/c-Al2O3/MoS2 heterostructure (Fig. 2a). The cross-sectional HR-TEM image of the heterostructure shows a stack consisting of single-crystalline Al, c-Al2O3 and MoS2 layers, from top to bottom, respectively. An atomically sharp interface is observed between c-Al2O3 and MoS2. The magnified images of the dashed green, orange and blue boxes in Fig. 2a (right) indicate single-crystalline Al, c-Al2O3 and MoS2, respectively. Figure 2b shows the selected area electron diffraction patterns of the dashed green, orange and blue boxes in Fig. 2a confirming the crystallinity of each stacked region. The line profiles (Fig. 2b, bottom) along the dashed green, orange and blue lines in Fig. 2a show periodic variability in the interatomic distance. The distances for Al, c-Al2O3 and MoS2 are derived to be 0.21 nm, 0.37 nm and 0.6 nm, respectively.
a, Schematic (left), cross-sectional HR-TEM (middle) and magnified atomic-resolution (right) images of an Al/c-Al2O3/MoS2 heterostructure. b, Selected area electron diffraction patterns (top) obtained from the dashed green, orange and blue boxes in a. Line intensity profiles (bottom) along the dashed green, orange and blue lines in a. c, HR-TEM image of a-Al2O3/MoS2 (left) and the important tunnelling contributions (right). d, HR-TEM image of c-Al2O3/MoS2 (left) and the important tunnelling contributions (right). e, Experimental gate leakage currents for 2D FETs with c-Al2O3 and a-Al2O3. f, The relationship between current and gate voltage. VTG, top-gate voltage; VBG, back-gate voltage. g, The relationship between EOT and dielectric thickness. h, Experimental gate leakage currents compared with EOT measured at standard operating gate voltages of 1 V. i, Noise power spectra as a function of frequency. j, Comparison of Dit values measured for Si devices and different 2D technologies. Scale bars, 5 nm (a, bottom row, middle); 2 nm (a, bottom row, right); 2 nm (c,d). TAT, trap-assisted tunnelling.
Two different types of Al2O3 dielectric materials, amorphous and crystalline, are transferred onto the MoS2 channel by the same integration method. The HR-TEM images of a-Al2O3/MoS2 are shown in Fig. 2c (left) and those of c-Al2O3/MoS2 are shown in Fig. 2d (left). The important tunnelling contributions of the a-Al2O3/MoS2 junctions are shown in Fig. 2c (right) and those of the c-Al2O3/MoS2 junctions are shown in Fig. 2d (right). Apart from direct tunnelling through a-Al2O3, trap-assisted tunnelling, which is observed in insulators containing a substantial number of defects, plays an important part. Conversely, these tunnelling currents are suppressed because c-Al2O3 has a wider bandgap and a smaller defect density. Figure 2e shows the experimental J values of c-Al2O3/MoS2 and a-Al2O3/MoS2, and c-Al2O3/MoS2 shows a J value that is about two orders of magnitude lower than that of a-Al2O3/MoS2.
A double-gate device with SiO2/Si as the global back gate and Al/c-Al2O3 as the top-gate stack is fabricated to measure the equivalent oxide thickness (EOT) accurately. The top-gate capacitance (CTG) is extracted experimentally as shown in Extended Data Fig. 6. The EOT is calculated to be 1.4 nm (Fig. 2f) using the equation EOT = 3.45/CTG (ref. 32). Furthermore, six devices with different EOTs are fabricated as shown in Extended Data Fig. 6. Figure 2g compares the EOT values for different dielectric thicknesses (td). The comparison of the dielectric constant with td is shown in Supplementary Fig. 2. As expected, EOT increases linearly with td for both a-Al2O3 (refs. 35,36,37,38,39) and c-Al2O3, which is consistent with the equation EOT = 3.9td/εd, where εd is the dielectric constant of Al2O3 (refs. 30,40). Specifically, c-Al2O3 shows the smallest EOT compared with other 2D devices based on Al2O3 reported so far and also demonstrates the potential for scaling down 2D FETs.
The J values of other typical amorphous oxide dielectrics20,21,32,41,42,43,44, c-Al2O3 dielectrics and crystalline dielectrics11,13,15 with varying EOTs are provided in Fig. 2h. Except for hBN, the crystalline dielectric materials with a well-defined interface have a smaller J than conventional amorphous oxides. As a result of the small bandgap and low permittivity, 2D hBN exhibits extremely high J values15. The c-Al2O3/MoS2 device shows a J value of 10−6 A cm−2, which is four orders of magnitude lower than the low-power requirement (10−2 A cm−2) by IRDS3,32. Moreover, the J values (EOT = 0.77 nm; Supplementary Fig. 3) are steady even when the temperature increases to about 403 K (Supplementary Fig. 4), indicating that the trap-assisted tunnelling phenomenon in amorphous dielectric materials is hardly seen in c-Al2O3. Another comparison between c-Al2O3/MoS2 and the mature Si-CMOS technology shows that J is five orders of magnitude lower than that of Al2O3/Si (ref. 20), suggesting great scaling-down potential for the c-Al2O3/MoS2 FETs.
The interface state density (Dit) is determined by the 1/f noise method30 as shown in Fig. 2i. Because of the well-defined interface between c-Al2O3 and MoS2, a reduced Dit of 8.4 × 109 cm−2 eV−1 is achieved, similar to the value measured by the capacitance–voltage method (Supplementary Fig. 5). The calculation details are described in the Methods. The comparison of the Dit values with those in the literature is presented in Fig. 2j, and the details are shown in Supplementary Table 3. Most of the Dit values of exfoliated MoS2 channels are high ranging from 5 × 1011 cm−2 eV−1 to 1013 cm−2 eV−1 for both conventional amorphous oxide dielectrics31,32,37,43,44,45,46,47,48,49 and crystalline dielectrics11,13,15. In our case, a Dit of 8.4 × 109 cm−2 eV−1 is achieved from c-Al2O3/MoS2, and it meets the IRDS requirement for low-standby-power CMOS and is comparable to that of Si/SiO2 (Dit ~ 109 cm−2 eV−1) (ref. 50).
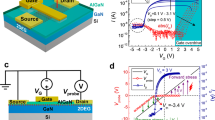
To study the electronic characteristics of the 2D FET based on c-Al2O3, a self-aligned MoS2 FET with 2 nm c-Al2O3 is fabricated by the vdW transfer method. The fabrication process of the self-aligned MoS2 FET is shown in Fig. 3a and Supplementary Fig. 6. Figure 3b shows the scanning electron microscopy (SEM) image of an array of the self-aligned MoS2 FETs. The width and length of the Al gate are 100 μm and 250 nm, respectively. A small air gap between Au and the Al gates, which ensures the complete insulation between the top gate and source or drain and the successful self-alignment process, is shown in Fig. 3c. The channel length is 300 nm (Lch = 300 nm). The cross-sectional TEM image of self-aligned MoS2 FET is shown in Fig. 3d. The vdW transfer method enables the fabrication of the complete FET stack by the self-alignment process, including the source, drain, dielectric and gate on the graphene/Ge donor wafer, and then it is transferred onto the channel materials in a one-step lamination process to produce the 2D FET with a good contact and dielectric interfaces48.
a, The fabrication process of a self-aligned c-Al2O3/MoS2 FET. b, SEM image of an array of self-aligned MoS2 FETs. c, Magnified SEM image of self-aligned MoS2 FET with a small air gap. d, Cross-sectional TEM image of self-aligned MoS2 FET. e,f, The output (e) and transfer (f) characteristics of a short-channel three-layered MoS2 FET. g,h, The output (g) and transfer (h) characteristics of a long-channel three-layered MoS2 FET. i, The dual-sweep transfer curve. j, Experimental SS values versus Ion/Ioff of MoS2 FETs with various dielectrics. Scale bars, 10 μm (b); 200 nm (c); 100 nm (d).
The output (drain current versus drain voltage, ID–VD) and transfer (drain current versus gate voltage, ID–VTG) characteristics of the MoS2 FET are shown in Fig. 3e,f. The output characteristics demonstrate promising current control and saturation. The drain current of the transfer curves shows a steep increase in the subthreshold region with a subthreshold swing (SS) of about 76 mV dec−1 and an on/off current ratio (Ion/Ioff) of 109. The output and transfer characteristics of the long-channel MoS2 FET are shown in Fig. 3g,h. A small SS of 61 mV dec−1 (Extended Data Fig. 7) close to the thermal limit of 60 mV dec−1 at 300 K is accomplished. A small hysteresis of 10 mV is also observed in the dual-sweep linear transfer curve in Fig. 3i, indicating a low trapping charge density in c-Al2O3. Moreover, a contrast experiment is performed using FETs containing c-Al2O3 and a-Al2O3 (Extended Data Fig. 8). The SS and hysteresis obtained from the device with c-Al2O3 (SS = 61 mV dec−1 and hysteresis of 40 mV) are notably lower than those of a-Al2O3 (SS = 120 mV dec−1 and hysteresis of 250 mV). Figure 3j shows the SS versus Ion/Ioff results of MoS2 FETs comprising different dielectrics10,11,12,13,27,30,31,32,33,37,42,44,46,47,48,49, and the details are shown in Supplementary Table 4. Specifically, the MoS2 FET using c-Al2O3 as gate dielectric delivers the best overall electrical performance such as low SS and high Ion/Ioff.
To further demonstrate the scalable fabrication of large-area top-gate FETs, the FET arrays are prepared on a 4-inch chemical vapour deposition(CVD)-MoS2 wafer. The photograph of the 4-inch CVD-MoS2/sapphire wafer with the top-gate FET arrays in Fig. 4a confirms that the complete FET stack is transferred to the MoS2/sapphire substrate. Figure 4b,c does not show any wrinkles or cracks. The typical output curves of these transistors demonstrate excellent electrostatic control of the channel because of the high-quality dielectrics (Fig. 4d). The transfer curves of 100 MoS2 FETs exhibit the typical n-type characteristics with excellent uniformity (Fig. 4e). The statistical distributions of the on/off current ratio (Ion/Ioff) and SS extracted from 100 devices are shown in Fig. 4f and further statistical distributions of the key parameters, including the threshold voltage, off-state current and on-state current are shown in Extended Data Fig. 9. Seventy percent of the devices show SS values in the range of 75–175 mV dec−1 and Ion/Ioff higher than 106, which are among the best for CVD-MoS2 FET. The value and distribution of SS for MoS2 transistor array can be markedly improved as wafer-scale single-crystalline MoS2 with excellent quality is available and used in the future. The uniformity and scalability of the c-Al2O3/graphene FETs shown in Extended Data Fig. 10 indicate the ability of the fabricating high-performance radio frequency transistors and other complex circuits with c-Al2O3 technique in the future.
a, Photograph of a 4-inch CVD-MoS2/sapphire wafer with top-gate FET arrays. b, Magnified optical image from the red box in a. c, Magnified optical image from the red box in b. d, The typical output curves of c-Al2O3/MoS2 FETs. e, Transfer curves of 100 MoS2 FETs. f, The statistic distributions of the on/off current ratio and SS from 100 devices. Scale bars, 200 μm (b); 50 μm (c).
In conclusion, we have demonstrated the fabrication of single-crystalline Al2O3 as the high-quality dielectric layer for the top-gate 2D transistors. This breakthrough will serve as the foundation for further advancements in the diversity, scalability and manufacturability of single-crystalline oxides, facilitating the seamless transition of 2D semiconductors from laboratories to industrial environments. The approach for the growth of wafer-scale single-crystalline aluminium and aluminium oxide can be further extended to other metals, and some single-crystalline oxides that were unattainable before can be synthesized for various applications. A notable advancement in this journey is the ability to scale up to the current silicon wafer diameter because the starting germanium for single-crystalline graphene synthesis can be epitaxially grown on silicon directly, which markedly broadens the potential for integration into established silicon-factory manufacturing processes. Meanwhile, the development of an automated debonding-transfer tool dedicated to low-dimensional materials, including single-crystalline dielectric oxide, 2D channel material and ultrathin metal electrode, is crucial to achieve the unique alignments between them for multifunctional 2D devices and is expected to substantially increase throughput, improve reproducibility and enhance the reliability of 2D integrated circuits. By constructing complex 2D integrated circuits, particularly for hetero-integration on mature Si-CMOS platforms, the immense potential of 2D materials can be fully unlocked and can lay the groundwork for the next generation of high-performance electronic devices.
Methods
Synthesis of single-crystalline Gr/Ge(110) template substrate
A p-type Ge(110) wafer was placed in the quartz tube in a CVD system evacuated to 0.1 Pa. After switching off the vacuum pump, the quartz tube was heated to 916 °C under a flow of H2 (30 sccm) and Ar (300 sccm). When the temperature of the quartz tube reached 916 °C, Gr growth proceeded under a flowing gas mixture of CH4 (0.8 sccm), H2 (70 sccm) and Ar (700 sccm) for 600 min. Then, CH4 and H2 were turned off, and the quartz tube cooled to room temperature under Ar (200 sccm). In this process, single-crystalline Gr/Ge(110) template substrate was synthesized successfully25.
Materials preparation
In the fabrication of a single MoS2 transistor, the MoS2 flakes were exfoliated mechanically and were dry-transferred to a 300-nm SiO2/p++ silicon substrate using the PF gel films (Gel Pak). In the batch fabrication of MoS2 transistors, the wafer-scale polycrystal monolayer CVD-grown MoS2 on sapphire was patterned into arrays by RIE (50 sccm O2, 30 W and 10 Pa) and photolithography. In the batch fabrication of graphene transistors, the CVD Gr grown on the Ge substrate was transferred to 300 nm SiO2/p++ silicon by PMMA-assisted transfer and patterned into arrays by RIE (50 sccm O2, 30 W and 10 Pa) and photolithography. All the 2D materials were vacuum annealed at 250 °C for 2 h to remove the photoresist and absorbents.
Device fabrication
An Al film with a thickness of 100 nm was deposited by an electron beam evaporation at 10−7 torr at a rate of 0.6 Å s−1. Electron beam lithography and photolithography and electron beam evaporation (Texas Instruments DE400) were used to define the Al nanoribbon electrodes on the Gr/Ge(110) substrate after the lift-off process. The top and side of Al nanoribbons were oxidized to form a-Al2O3 in an ambient environment. Afterwards, 10–15 nm thick Au was deposited on the Gr/Ge(110) template to form the self-aligned source (S)/drain (D) at a rate of 0.1 Å s−1. Then, the dry polyvinyl alcohol (PVA) film was laminated on the self-aligned structure at 90 °C for 3 min by a PDMS stamp. In an anaerobic glovebox (0.2 ppm oxygen), the self-aligned device stack together with PVA was peeled off and physically laminated on top of the mechanically exfoliated few-layer MoS2 (or wafer-scale CVD-MoS2 on sapphire) at 90 °C for 3 min by a PDMS stamp using a microscopic alignment system. After the transfer process, c-Al2O3 was formed as the gate dielectric on Al in an oxygen-deficient environment (0.2 ppm O2). Finally, the PVA film was dissolved in dimethyl sulfoxide (DMSO). For the metal–insulator–metal (MIM) capacitor, the Cr/Au (10/50 nm) bottom electrodes were deposited on the sapphire substrate to eliminate the parasitic capacitance.
Cross-sectional TEM sample preparation and characterization
The patterned Au/Al bilayer with a thickness of 20 nm/100 nm was deposited on the Gr/Ge(110) substrate by electron beam evaporation. The dry PVA film was laminated on top of the Au/Al patterns at 90 °C for 3 min by a PDMS stamp. The Au/Al patterns together with PVA were peeled off from the Gr/Ge(110) substrate and attached to a PDMS stamp. To change the thickness of c-Al2O3, the Au/Al patterns transferred onto PVA were exposed to an oxygen-deficient environment for different durations after the peeling process. Finally, the Au/Al/c-Al2O3 patterns were transferred on the MoS2, SiO2 and sapphire substrates at 90 °C for 3 min by a PDMS stamp on a microscopic alignment system. The PVA film was dissolved in dimethyl sulfoxide (DMSO). The entire transfer process was carried out in an anaerobic glovebox (0.2 ppm oxygen). A 20-nm Au protection layer was deposited directly on the whole substrate, which also serves as the conduction layer for the subsequent focused-ion-beam process. The cross-sectional sample was prepared by focused-ion-beam process (Helios G4 UX, Thermo Fischer) with 30 keV Ga+ and the sample was examined by TEM (aberration-corrected JEM-ARM300F) at 300 kV.
Device and materials characterization
Raman scattering was performed on the HORIBA Jobin Yvon HR800 with a 514-nm excitation laser to monitor the quality of CVD Gr/Ge and MoS2. The surface topography of Gr/Ge was assessed by atomic force microscopy (Multimode 8, Bruker) using the tapping mode. The surface topography and structure of the devices were examined by SEM (Zeiss SUPRA 55). The electrical measurements were performed under ambient conditions at room temperature in the dark on the semiconductor parameter analyser (Agilent B1500A) and commercial high-resolution photoelectric scanner (MStarter200).
EOT extraction
The EOT was estimated based on the linear relationship of the Dirac point voltage (VTG, Dirac) drift of the top-gate graphene transistor or the threshold voltage (VTH) drift of the top-gate MoS2 transistor as a function of the back-gate voltage (VBG) (ref. 32) by the following equation:
where 3.45 μF cm−2 is the gate capacitance of 1 nm SiO2, CTG is the top-gate capacitance and CBG is the back-gate capacitance of 300 nm SiO2.
Interface states extraction
The interface state, Dit, was extracted according to the 1/f noise method30 using the following equation:
where SI is spectral density, gm is the gate transconductance, Svfb is the flat band voltage spectral density, q is the electronic charge, KB is Boltzmann’s constant, T is the temperature, W and L are the width and the length of the channel, respectively, and Cox is the gate capacitance per unit area.
The interface state, Dit, was extracted according to the C–V method48 using the following equation:
where q is the electronic charge, Cox is the gate capacitance per unit area, CLH and CHF are the measured quasistatic (low-frequency) and high-frequency depletion capacitance per unit area, respectively.
Data availability
Source data are provided with this paper.
Change history
06 September 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41586-024-08001-y
References
Liu, C. et al. Two-dimensional materials for next-generation computing technologies. Nat. Nanotechnol. 15, 545–557 (2020).
Liu, Y. et al. Promises and prospects of two-dimensional transistors. Nature 591, 43–53 (2021).
Das, S. et al. Transistors based on two-dimensional materials for future integrated circuits. Nat. Electron. 4, 786–799 (2021).
Li, W. et al. Approaching the quantum limit in two-dimensional semiconductor contacts. Nature 613, 274–279 (2023).
Illarionov, Y. Y. et al. Insulators for 2D nanoelectronics: the gap to bridge. Nat. Commun. 11, 3385 (2020).
Yang, S. et al. Gate dielectrics integration for 2D electronics: challenges, advances, and outlook. Adv. Mater. 35, 2207901 (2023).
IRDS. International Roadmap for Devices and Systems: 2021 Update. Executive Summary (IEEE, 2021).
Chen, X. et al. Wafer-scale functional circuits based on two dimensional semiconductors with fabrication optimized by machine learning. Nat. Commun. 12, 5953 (2021).
Tang, J. et al. Low power flexible monolayer MoS2 integrated circuits. Nat. Commun. 14, 3633 (2023).
Yang, X. et al. Highly reproducible van der Waals integration of two-dimensional electronics on the wafer scale. Nat. Nanotechnol. 18, 471–478 (2023).
Illarionov, Y. Y. et al. Ultrathin calcium fluoride insulators for two-dimensional field-effect transistors. Nat. Electron. 2, 230–235 (2019).
Liu, K. et al. A wafer-scale van der Waals dielectric made from an inorganic molecular crystal film. Nat. Electron. 4, 906–913 (2021).
Huang, J.-K. et al. High-κ perovskite membranes as insulators for two-dimensional transistors. Nature 605, 262–267 (2022).
Vu, Q. A. et al. Near-zero hysteresis and near-ideal subthreshold swing in h-BN encapsulated single-layer MoS2 field-effect transistors. 2D Mater. 5, 031001 (2018).
Britnell, L. et al. Electron tunneling through ultrathin boron nitride crystalline barriers. Nano Lett. 12, 1707–1710 (2012).
Zhang, Y. et al. A single-crystalline native dielectric for two-dimensional semiconductors with an equivalent oxide thickness below 0.5 nm. Nat. Electron. 5, 643–649 (2022).
Low, C. G., Zhang, Q., Hao, Y. & Ruoff, R. S. Graphene field effect transistors with mica as gate dielectric layers. Small 10, 4213–4218 (2014).
Zavabeti, A. et al. A liquid metal reaction environment for the room-temperature synthesis of atomically thin metal oxides. Science 358, 332–335 (2017).
Zhang, B. Y. et al. Hexagonal metal oxide monolayers derived from the metal-gas interface. Nat. Mater. 20, 1073–1078 (2021).
Guha, S. et al. High-quality aluminum oxide gate dielectrics by ultra-high-vacuum reactive atomic-beam deposition. J. Appl. Phys. 90, 512–514 (2001).
Gusev, E. P. et al. Ultrathin 338 high-K gate stacks for advanced CMOS devices. In International Electron Devices Meeting (IEDM). Technical Digest (Cat. No.01CH37224) 20.1.1–20.1.4 (IEEE, 2001).
Ren, F. et al. Van der Waals epitaxy of nearly single-crystalline nitride films on amorphous graphene-glass wafer. Sci. Adv. 7, eabf5011 (2021).
Kim, J. et al. Principle of direct van der Waals epitaxy of single-crystalline films on epitaxial graphene. Nat. Commun. 5, 4836 (2014).
Periwal, P. et al. Catalytically mediated epitaxy of 3D semiconductors on van der Waals substrates. Appl. Phys. Rev. 7, 031402 (2020).
Liu, G. et al. Graphene-assisted metal transfer printing for wafer-scale integration of metal electrodes and two-dimensional materials. Nat. Electron. 5, 275–280 (2022).
McPherson, J., Kim, J., Shanware, A., Mogul, H. & Rodriguez, J. Proposed universal relationship between dielectric breakdown and dielectric constant. In 2002 IEEE International Electron Devices Meeting (IEDM) 633–636 (IEEE, 2002).
Qian, Q. et al. Improved gate dielectric deposition and enhanced electrical stability for single-layer MoS2 MOSFET with an AlN interfacial layer. Sci. Rep. 6, 27676 (2016).
Dicks, O. A. & Shluger, A. L. Theoretical modeling of charge trapping in crystalline and amorphous Al2O3. J. Phys. Condens. Matter 29, 314005 (2017).
Lee, B. H., Jeon, Y., Zawadzki, K., Qi, W. J. & Lee, J. Effects of interfacial layer growth on the electrical characteristics of thin titanium oxide films on silicon. Appl. Phys. Lett. 74, 3143–3145 (1999).
Lu, Z. et al. Wafer-scale high-κ dielectrics for two-dimensional circuits via van der Waals integration. Nat. Commun. 14, 2340 (2023).
Zhu, Y. et al. Monolayer molybdenum disulfide transistors with single-atom-thick gates. Nano Lett. 18, 3807–3813 (2018).
Li, W. et al. Uniform and ultrathin high-κ gate dielectrics for two-dimensional electronic devices. Nat. Electron. 2, 563–571 (2019).
Luo, P. et al. Molybdenum disulfide transistors with enlarged van der Waals gaps at their dielectric interface via oxygen accumulation. Nat. Electron. 5, 849–858 (2022).
Wen, C. et al. Dielectric properties of ultrathin CaF2 ionic crystals. Adv. Mater. 32, 2002525 (2020).
English, C. D., Smithe, K. K. H., Xu, R. J. & Pop, E. Approaching ballistic transport in monolayer MoS2 transistors with self-aligned 10 nm top gates. In 2016 IEEE International Electron Devices Meeting (IEDM) 5.6.1–5.6.4 (IEEE, 2016).
McClellan, C. J., Mleczko, M. J., Smithe, K. K. H., Nishi, Y. & Pop, E. WTe2 as a two-dimensional (2D) metallic contact for 2D semiconductors. In 2016 74th Annual Device Research Conference (Drc) 1–2 (IEEE, 2016).
Wang, X. et al. Improved integration of ultra-thin high-k dielectrics in few-layer MoS2 FET by remote forming gas plasma pretreatment. Appl. Phys. Lett. 110, 053110 (2017).
Patel, K. A., Grady, R. W., Smithe, K. K. H., Pop, E. & Sordan, R. Ultra-scaled MoS2 transistors and circuits fabricated without nanolithography. 2D Mater. 7, 015018 (2020).
Nam, T., Seo, S. & Kim, H. Atomic layer deposition of a uniform thin film on two-dimensional transition metal dichalcogenides. J. Vac. Sci. Technol. A 38, 030803 (2020).
Wong, H. & Iwai, H. On the scaling of subnanometer EOT gate dielectrics for ultimate nano CMOS technology. Microelectron. Eng. 138, 57–76 (2015).
Brar, B., Wilk, G. D. & Seabaugh, A. C. Direct extraction of the electron tunneling effective mass in ultrathin SiO2. Appl. Phys. Lett. 69, 2728–2730 (1996).
Zou, X. et al. Interface engineering for high-performance top-gated MoS2 field-effect transistors. Adv. Mater. 26, 6255–6261 (2014).
Park, J. H. et al. Atomic layer deposition of Al2O3 on WSe2 functionalized by titanyl phthalocyanine. ACS Nano 10, 6888–6896 (2016).
Cheng, L. et al. Sub−10 nm tunable hybrid dielectric engineering on MoS2 for two-dimensional material-based devices. ACS Nano 11, 10243–10252 (2017).
Zhu, W. et al. Electronic transport and device prospects of monolayer molybdenum disulphide grown by chemical vapour deposition. Nat. Commun. 5, 3087 (2014).
Wang, J. et al. Integration of high-k oxide on MoS2 by using ozone pretreatment for high-performance MoS2 top-gated transistor with thickness-dependent carrier scattering investigation. Small 11, 5932–5938 (2015).
Zou, X. et al. Dielectric engineering of a boron nitride/hafnium oxide heterostructure for high-performance 2D field effect transistors. Adv. Mater. 28, 2062–2069 (2016).
Wang, L. et al. A general one-step plug-and-probe approach to top-gated transistors for rapidly probing delicate electronic materials. Nat. Nanotechnol. 17, 1206–1213 (2022).
Uchiyama, H., Maruyama, K., Chen, E., Nishimura, T. & Nagashio, K. A monolayer MoS2 FET with an EOT of 1.1 nm achieved by the direct formation of a high-κ Er2O3 insulator through thermal evaporation. Small 19, 2207394 (2023).
Boutchacha, T., Ghibaudo, G., Guégan, G. & Skotnicki, T. Low frequency noise characterization of 0.18 μm Si CMOS transistors. Microelectron. Reliab. 37, 1599–1602 (1997).
Acknowledgements
We thank the National Natural Science Foundation of China (grant nos. 51925208 and 62122082), the National Key R&D Program of China (grant nos. 2022YFB3204800 and 2022YFB4400100), the Science and Technology Commission of Shanghai Municipality (grant no. 21JC1406100), the CAS Project for Young Scientists in Basic Research (grant no. YSBR-081) and the City University of Hong Kong Donation Research Grants (grant nos. 922061 and DON-RMG 9229021). Some of the experiments were carried out at the Fudan Nanofabrication Laboratory. We thank F. Ding and P. Li from Shanghai Institute of Microsystem and Information Technology (SIMIT), Chinese Academy of Sciences, for their theoretical support.
Author information
Authors and Affiliations
Contributions
Z.D. and Z.T. conceived and supervised the project. D.Z. fabricated the devices and performed the measurements. D.Z. and Z.Z. contributed to the transfer-printing method and growth of graphene. D.Z., Z.T., Y.M., Z.X., M.Z., P.K.C. and Z.D. contributed to the data analysis. Z.T., D.Z., Z.D. and P.K.C. co-wrote the paper. All authors discussed the results and contributed to the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Epitaxial single-crystalline Al (111) on Gr/Ge (110) substrate.
a, Cross-sectional HRTEM image of Al/Gr/Ge (110) interface. b, Magnified cross-sectional HRTEM of Al/Gr/Ge (110) interface from the red boxes in (a). c, and d, Magnified cross-sectional HRTEM of single-crystalline Al (111) and single-crystalline Ge (110) remote epitaxial template from the red boxes in (b), respectively. e, EDS mapping images of Al/Gr/Ge interface. f, AFM thickness of single crystalline Al layer grown on Gr/Ge (110) substrate obtained from 28 random points on the wafer. g, High-resolution XRD of Al(111)/Gr/Ge(110) heterostructure obtained from 15 random points on the wafer.
Extended Data Fig. 2 The epitaxial relationship between c-Al2O3 (0001) and Al (111).
a, Cross-sectional HRTEM of Al(111)/Al2O3(0001). b, Selected area electron diffraction patterns of Al2O3(0001) and Al(111). c, Side view of Al(111)/ Al2O3(0001).
Extended Data Fig. 3 Schematic and optical images of the graphene-assisted Al/c-Al2O3 water-free transfer printing process.
a, Schematic of the water-free transfer process of Al/c-Al2O3 patterns under an oxygen-deficient environment in a glovebox. The process starts with a clean CVD Gr/Ge substrate; followed by defining Al patterns by electron beam lithography (EBL)/photolithography, depositing Al by electron beam evaporation and lifting off; first printing dry PVA film on Al patterns using a PDMS stamp at 90 °C; second peeling off Al patterns from Gr/Ge substrate using dry PVA film at room temperature in a glovebox; second transferring and printing PVA/Al/c-Al2O3 on target substrate using a PDMS stamp at 90 °C; finally, the PVA film is dissolved by dimethyl sulfoxide (DMSO). b, Optical image of Al/c-Al2O3 patterns transferred onto a 4-inch PVA film. c, Optical image of Al/c-Al2O3 patterns transferred onto a 4-inch Si wafer. d, Magnified optical image of Al/c-Al2O3 patterns from the red box in (c).
Extended Data Fig. 4 The reuse of Gr/Ge template substrate.
a, and b, Schematic and optical images of reusing Gr/Ge template substrate for Al/c-Al2O3 patterns transfer. c, AFM image of pristine Gr/Ge substrate with a surface roughness of 0.261 nm from the blue box in (b). d, and e, Raman line mapping and area mapping of pristine Gr/Ge substrate. f, AFM image of Gr/Ge substrate from the red box in (b) after first peeling with the surface roughness of 0.299 nm. g, and h, Raman line mapping and area mapping of Gr/Ge substrate from the red box in (b) after first peeling.
Extended Data Fig. 5 Cross-sectional HRTEM images of vdW-transferred Al/c-Al2O3 with different thickness on the sapphire substrate.
a-d, The cross-sectional HRTEM images and corresponding enlarged images from the red box of 2 nm, 3 nm, 6 nm, and 10 nm c-Al2O3 by exposing Al to an oxygen-deficient environment for 1 h, 2 h, 5 h, and 12 h, respectively.
Extended Data Fig. 6 Double-gate measurement to extract EOT.
a, A schematic of the double-gate graphene transistor model. CTG is the top gate capacitance of c-Al2O3, CBG (0.0115 μF cm−2) is the back gate capacitance of 300 nm SiO2, CQ is the quantum capacitance of graphene. b, and c, Transfer curves of a typical top gate graphene transistor with c-Al2O3 as gate dielectrics and corresponding transfer curves under different VBG, VD = 0.1 V. d-g, Dirac point voltage (VTG, Dirac) versus VBG in top gate graphene transistors. From the linear fitting, EOT of 1 nm, 1.46 nm, 1.64 nm, and 1.67 nm are obtained respectively. h, Liner fitting of threshold voltage of a top gate MoS2 transistor, EOT of 3.3 nm is obtained. i, C-V characteristics for MIM devices with 10 nm c-Al2O3 under different frequencies, EOT of 4 nm is obtained.
Extended Data Fig. 7 SS distribution and hysteresis of vdW-transferred c-Al2O3 top-gate MoS2 transistors.
a, Transfer curves of top-gate MoS2 transistor. b, SS distribution as a function of channel current in a. The dashed line represents the thermal limit of SS at room temperature (60 mV/dec). c, The double-sweep transfer curves with different step size and sweeping rates of MoS2 transistor using the transferred c-Al2O3/Al gate stack. d, Hysteresis versus sweep rate.
Extended Data Fig. 8 Electrical performance of a-Al2O3 and c-Al2O3 top-gate MoS2 transistors.
a, and b, Transfer and output curves of ALD a-Al2O3 top-gate MoS2 transistors. c, The double-sweep transfer curves with different gate-voltage ranges of ALD a-Al2O3 top-gate MoS2 transistors. d, and e, Transfer and output curves of vdW-transferred c-Al2O3 top-gate MoS2 transistors. f, Enlarged double-sweep transfer curves with different gate-voltage ranges of vdW-transferred c-Al2O3 top-gate MoS2 transistors. The top-gate MoS2 transistors with vdW-transferred c-Al2O3 dielectric and Au contracts show a higher on/off ratio (1.05 × 108), smaller subthreshold swing (SS ≈ 61 mV/dec) and hysteresis (40 mV). By contrast, the devices with ALD a-Al2O3 and deposited Au contacts show a notably smaller on/off ratio (1.4 × 107), larger SS (120 mV/dec), and hysteresis (250 mV).
Extended Data Fig. 9 Electrical performance of CVD-MoS2 transistor array.
Histogram and Lorentz’s distribution fits of a, subthreshold swing SS, b, threshold voltage, Vth, c, on/off ratio ION/IOFF, d, off-state current IOFF, and e, on-state current ION extracted from the transfer curves of 100 devices. According to Lorentz distribution fittings, the statistical results of SS, Vth, ION/IOFF, IOFF, and ION show centralized distributions with average values of 121.8 mV/dec, 0.63 V, 3.2 × 106, 7 × 10−14 A, 2 × 10−7 A, respectively. The electrical performance and uniformity of MoS2 transistors can be further improved by using single crystalline CVD MoS2 wafer in the future.
Extended Data Fig. 10 Electrical performance of CVD-grown graphene transistor array.
a, Optical image of the top-gate graphene transistor array with vdW-transferred c-Al2O3 dielectric and Au contracts. b, SEM image of a single graphene transistor in a. c and d, Transfer and output curves of a typical graphene transistor. e, Transfer curves of 43 graphene transistors in the array, VD = 0.1 V. f, Histogram and Lorentz’s distribution fits of Dirac point voltage, the Dirac point voltage shows centralized distribution with average values of 0.13 V.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–6 and Supplementary Tables 1–5.
Supplementary Video 1
Peeling process of the 4-inch patterned Al film from the Gr/Ge substrate using PVA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeng, D., Zhang, Z., Xue, Z. et al. Single-crystalline metal-oxide dielectrics for top-gate 2D transistors. Nature 632, 788–794 (2024). https://doi.org/10.1038/s41586-024-07786-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07786-2
- Springer Nature Limited