Abstract
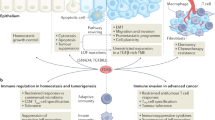
The immune system responds to cancer in two main ways. First, there are prewired responses involving myeloid cells, innate lymphocytes and innate-like adaptive lymphocytes that either reside in premalignant tissues or migrate directly to tumours, and second, there are antigen priming-dependent responses, in which adaptive lymphocytes are primed in secondary lymphoid organs before homing to tumours. Transforming growth factor-β (TGFβ) — one of the most potent and pleiotropic regulatory cytokines — controls almost every stage of the tumour-elicited immune response, from leukocyte development in primary lymphoid organs to their priming in secondary lymphoid organs and their effector functions in the tumour itself. The complexity of TGFβ-regulated immune cell circuitries, as well as the contextual roles of TGFβ signalling in cancer cells and tumour stromal cells, necessitates the use of rigorous experimental systems that closely recapitulate human cancer, such as autochthonous tumour models, to uncover the underlying immunobiology. The diverse functions of TGFβ in healthy tissues further complicate the search for effective and safe cancer therapeutics targeting the TGFβ pathway. Here we discuss the contextual complexity of TGFβ signalling in tumour-elicited immune responses and explain how understanding this may guide the development of mechanism-based cancer immunotherapy.





Similar content being viewed by others
References
Batlle, E. & Massague, J. Transforming growth factor-beta signaling in immunity and cancer. Immunity 50, 924–940 (2019).
Derynck, R., Turley, S. J. & Akhurst, R. J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34 (2021).
van den Bulk, J., de Miranda, N. & Ten Dijke, P. Therapeutic targeting of TGF-β in cancer: hacking a master switch of immune suppression. Clin. Sci. 135, 35–52 (2021).
David, C. J. & Massague, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 19, 419–435 (2018).
Li, M. O., Wan, Y. Y., Sanjabi, S., Robertson, A. K. & Flavell, R. A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24, 99–146 (2006).
Sun, T. et al. TGFbeta2 and TGFbeta3 isoforms drive fibrotic disease pathogenesis. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abe0407 (2021).
Shi, M. et al. Latent TGF-beta structure and activation. Nature 474, 343–349 (2011).
Yang, Z. et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J. Cell Biol. 176, 787–793 (2007).
Aluwihare, P. et al. Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J. Cell Sci. 122, 227–232 (2009).
Dong, X. et al. Force interacts with macromolecular structure in activation of TGF-beta. Nature 542, 55–59 (2017).
Campbell, M. G. et al. Cryo-EM reveals integrin-mediated TGF-β activation without release from latent TGF-β. Cell 180, 490–501 e416 (2020).
Nagano, Y. et al. Arkadia induces degradation of SnoN and c-Ski to enhance transforming growth factor-beta signaling. J. Biol. Chem. 282, 20492–20501 (2007).
Levy, L. et al. Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation. Mol. Cell Biol. 27, 6068–6083 (2007).
Gori, I. et al. Mutations in SKI in Shprintzen-Goldberg syndrome lead to attenuated TGF-beta responses through SKI stabilization. Elife https://doi.org/10.7554/eLife.63545 (2021).
Zhang, L. et al. A transforming growth factor beta-induced Smad3/Smad4 complex directly activates protein kinase A. Mol. Cell Biol. 24, 2169–2180 (2004).
Derynck, R. & Budi, E. H. Specificity, versatility, and control of TGF-beta family signaling. Sci. Signal. https://doi.org/10.1126/scisignal.aav5183 (2019).
Ouyang, W. et al. TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity 39, 335–346 (2013).
Ouyang, W., Beckett, O., Ma, Q. & Li, M. O. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32, 642–653 (2010).
Oh, S. A. et al. Foxp3-independent mechanism by which TGF-beta controls peripheral T cell tolerance. Proc. Natl Acad. Sci. USA 114, E7536–E7544 (2017).
Li, M. O., Sanjabi, S. & Flavell, R. A. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455–471 (2006).
Marie, J. C., Liggitt, D. & Rudensky, A. Y. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25, 441–454 (2006).
Takimoto, T. et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J. Immunol. 185, 842–855 (2010).
Gu, A. D., Wang, Y., Lin, L., Zhang, S. S. & Wan, Y. Y. Requirements of transcription factor Smad-dependent and -independent TGF-beta signaling to control discrete T-cell functions. Proc. Natl Acad. Sci. USA 109, 905–910 (2012).
Cattley, R. T., Lee, M., Boggess, W. C. & Hawse, W. F. Transforming growth factor beta (TGF-beta) receptor signaling regulates kinase networks and phosphatidylinositol metabolism during T-cell activation. J. Biol. Chem. 295, 8236–8251 (2020).
Prado, D. S. et al. Synergistic and additive interactions between receptor signaling networks drive the regulatory T cell versus T helper 17 cell fate choice. J. Biol. Chem. 297, 101330 (2021). The studies by Cattley et al. (2020) and Prado et al. (2021) demonstrate a biochemical mechanism by which TGFβ regulates TCR signalling in the absence or presence of the proinflammatory cytokine IL-6 in CD4+ T cells.
Gorelik, L., Constant, S. & Flavell, R. A. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 195, 1499–1505 (2002).
Gorelik, L., Fields, P. E. & Flavell, R. A. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 165, 4773–4777 (2000).
Heath, V. L., Murphy, E. E., Crain, C., Tomlinson, M. G. & O’Garra, A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur. J. Immunol. 30, 2639–2649 (2000).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Zhang, S. et al. Reversing SKI-SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature 551, 105–109 (2017).
Liu, M. et al. TGF-beta suppresses type 2 immunity to cancer. Nature 587, 115–120 (2020). This study reveals T helper cells as a crucial target of TGFβ-induced tumour immune tolerance in a transgenic model of breast cancer, blockade of which results in a TH2 cell-mediated tissue-level cancer defence response associated with tumour vasculature remodelling.
Jiao, S. et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177–1190 e1113 (2019). This study shows that blocking TGFβ synergizes with anti-CTLA4 and anti-PD1 treatment to suppress bone metastasis in a murine model of castration-resistant prostate cancer, in association with decreased TH17 cell, but enhanced TH1 cell, differentiation.
Gao, S., Hsu, T. W. & Li, M. O. Immunity beyond cancer cells: perspective from tumor tissue. Trends Cancer 7, 1010–1019 (2021).
Perez, L. G. et al. TGF-beta signaling in Th17 cells promotes IL-22 production and colitis-associated colon cancer. Nat. Commun. 11, 2608 (2020). This study demonstrates that TGFβ signalling sustains TH17 cell differentiation and IL-22 production, which promotes tumour development in a murine model of colitis-associated colon cancer.
Rizzo, A. et al. Smad7 expression in T cells prevents colitis-associated cancer. Cancer Res. 71, 7423–7432 (2011).
Rizzo, A. et al. Smad7 induces plasticity in tumor-infiltrating Th17 cells and enables TNF-alpha-mediated killing of colorectal cancer cells. Carcinogenesis 35, 1536–1546 (2014).
Malmberg, K. J. et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J. Clin. Invest. 110, 1515–1523 (2002).
Syu, L. J. et al. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am. J. Pathol. 181, 2114–2125 (2012).
DeNardo, D. G. et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91–102 (2009).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Cui, C. et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell 184, 6101–6118 e6113 (2021).
DiToro, D. et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science https://doi.org/10.1126/science.aao2933 (2018).
Marshall, H. D. et al. The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. Elife 4, e04851 (2015).
Liu, Y. et al. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 9, 632–640 (2008).
Zheng, Y. et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 (2010).
Schlenner, S. M., Weigmann, B., Ruan, Q., Chen, Y. & von Boehmer, H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J. Exp. Med. 209, 1529–1535 (2012).
Chen, W. et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Tone, Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 9, 194–202 (2008).
Hindley, J. P. et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 71, 736–746 (2011).
Malchow, S. et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339, 1219–1224 (2013).
Ahmadzadeh, M. et al. Tumor-infiltrating human CD4+ regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aao4310 (2019).
Xydia, M. et al. Common clonal origin of conventional T cells and induced regulatory T cells in breast cancer patients. Nat. Commun. 12, 1119 (2021).
Zhou, L. et al. TGF-beta-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240 (2008).
Gutcher, I. et al. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity 34, 396–408 (2011).
Sledzinska, A. et al. TGF-beta signalling is required for CD4+ T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol. 11, e1001674 (2013).
Donkor, M. K., Sarkar, A. & Li, M. O. Tgf-beta1 produced by activated CD4+ T cells antagonizes T cell surveillance of tumor development. Oncoimmunology 1, 162–171 (2012).
Dodagatta-Marri, E. et al. alpha-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by alpha-TGFbeta antibody to promote durable rejection and immunity in squamous cell carcinomas. J. Immunother. Cancer 7, 62 (2019).
Johnson, L. D. & Jameson, S. C. TGF-beta sensitivity restrains CD8+ T cell homeostatic proliferation by enforcing sensitivity to IL-7 and IL-15. PLoS ONE 7, e42268 (2012).
Arumugam, V. et al. TCR signaling intensity controls CD8+ T cell responsiveness to TGF. -beta. J. Leukoc. Biol. 98, 703–712 (2015).
Brownlie, R. J. et al. Resistance to TGFbeta suppression and improved anti-tumor responses in CD8+ T cells lacking PTPN22. Nat. Commun. 8, 1343 (2017).
Stephen, T. L. et al. Transforming growth factor beta-mediated suppression of antitumor T cells requires FoxP1 transcription factor expression. Immunity 41, 427–439 (2014).
Mackay, L. K. et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity 43, 1101–1111 (2015).
Thomas, D. A. & Massague, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005).
Park, B. V. et al. TGFbeta1-mediated SMAD3 enhances PD-1 expression on antigen-specific T cells in cancer. Cancer Discov. 6, 1366–1381 (2016).
Gorelik, L. & Flavell, R. A. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat. Med. 7, 1118–1122 (2001).
Gunderson, A. J. et al. TGFbeta suppresses CD8+ T cell expression of CXCR3 and tumor trafficking. Nat. Commun. 11, 1749 (2020). This study reveals that blockade of TGFβ signalling in CD8+ T cells synergizes with chemoradiotherapy to suppress tumour growth in a transplantable tumour model, which is associated with increased CXCR3 expression and CD8+ T cell tumour trafficking.
Luo, C. T., Liao, W., Dadi, S., Toure, A. & Li, M. O. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature 529, 532–536 (2016).
Dodagatta-Marri, E. et al. Integrin alphavbeta8 on T cells suppresses anti-tumor immunity in multiple models and is a promising target for tumor immunotherapy. Cell Rep. 36, 109309 (2021).
Laine, A. et al. Regulatory T cells promote cancer immune-escape through integrin alphavbeta8-mediated TGF-beta activation. Nat. Commun. 12, 6228 (2021). The studies by Dodagatta-Marri et al. (2021) and Laine et al. (2021) demonstrate that the Treg cell-expressed integrin αVβ8 promotes TGFβ1 activation and suppresses cancer immunosurveillance in transplantable tumour models and that αVβ8 is a potential immuno-oncology target.
de Streel, G. et al. Selective inhibition of TGF-beta1 produced by GARP-expressing Tregs overcomes resistance to PD-1/PD-L1 blockade in cancer. Nat. Commun. 11, 4545 (2020). This study shows that Treg cell-expressed GARP promotes tumour immune escape, which can be targeted in synergy with anti-PD1 to inhibit tumour growth in a transplantable tumour model.
Takasaka, N. et al. Integrin alphavbeta8-expressing tumor cells evade host immunity by regulating TGF-beta activation in immune cells. JCI Insight https://doi.org/10.1172/jci.insight.122591 (2018).
Seed, R. I. et al. A tumor-specific mechanism of Treg enrichment mediated by the integrin alphavbeta8. Sci. Immunol. https://doi.org/10.1126/sciimmunol.abf0558 (2021).
Chou, C. K. et al. Cell-intrinsic abrogation of TGF-beta signaling delays but does not prevent dysfunction of self/tumor-specific CD8 T cells in a murine model of autochthonous prostate cancer. J. Immunol. 189, 3936–3946 (2012).
Bendle, G. M., Linnemann, C., Bies, L., Song, J. Y. & Schumacher, T. N. Blockade of TGF-beta signaling greatly enhances the efficacy of TCR gene therapy of cancer. J. Immunol. 191, 3232–3239 (2013).
Zhong, W. et al. Comparison of the molecular and cellular phenotypes of common mouse syngeneic models with human tumors. BMC Genomics 21, 2 (2020).
Mani, V. et al. Migratory DCs activate TGF-beta to precondition naive CD8+ T cells for tissue-resident memory fate. Science https://doi.org/10.1126/science.aav5728 (2019).
Hirai, T. et al. Competition for active TGFbeta cytokine allows for selective retention of antigen-specific tissue- resident memory T cells in the epidermal niche. Immunity 54, 84–98 e85 (2021).
Ferreira, C. et al. Type 1 Treg cells promote the generation of CD8+ tissue-resident memory T cells. Nat. Immunol. 21, 766–776 (2020). This study reveals that T-bet-expressing type 1 Treg cells are recruited in close proximity to CD8+ T cells, and can promote differentiation of tissue-resident memory T cells via αVβ8-mediated activation of TGFβ1.
Hu, Y., Lee, Y. T., Kaech, S. M., Garvy, B. & Cauley, L. S. Smad4 promotes differentiation of effector and circulating memory CD8 T cells but is dispensable for tissue-resident memory CD8 T cells. J. Immunol. 194, 2407–2414 (2015).
Wu, B. et al. The SKI proto-oncogene restrains the resident CD103+CD8+ T cell response in viral clearance. Cell Mol. Immunol. 18, 2410–2421 (2021).
Igalouzene, R. et al. SMAD4 TGF-beta-independent function preconditions naive CD8+ T cells to prevent severe chronic intestinal inflammation. J. Clin. Invest. https://doi.org/10.1172/JCI151020 (2022).
Le Floc’h, A. et al. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 204, 559–570 (2007).
Hoffmann, J. C. & Schon, M. P. Integrin alphaE(CD103)beta7 in epithelial cancer. Cancers https://doi.org/10.3390/cancers13246211 (2021).
Boutet, M. et al. TGFbeta signaling Intersects with CD103 integrin signaling to promote T-lymphocyte accumulation and antitumor activity in the lung tumor microenvironment. Cancer Res. 76, 1757–1769 (2016). This study shows that TGFβ promotes CD103-dependent CD8+ T cell adhesion, signalling and antitumour functions in a human lung tumour slice culture system, supporting a positive role for TGFβ in epithelial cancer immunity.
Miller, C. H. et al. Eomes identifies thymic precursors of self-specific memory-phenotype CD8(+) T cells. Nat. Immunol. 21, 567–577 (2020).
Kim, H. J., Verbinnen, B., Tang, X., Lu, L. & Cantor, H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 467, 328–332 (2010).
McCarron, M. J. & Marie, J. C. TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity. J. Clin. Invest. 124, 4375–4386 (2014).
Mishra, S. et al. TGF-beta and Eomes control the homeostasis of CD8+ regulatory T cells. J. Exp. Med. https://doi.org/10.1084/jem.20200030 (2021). This study demonstrates that TGFβ cooperates with EOMES to promote homeostasis of regulatory CD8+ T cells, deficiency of which results in lethal autoimmunity.
Chu, F. et al. CXCR5+CD8+ T cells are a distinct functional subset with an antitumor activity. Leukemia 33, 2640–2653 (2019).
McDonald, B. D., Jabri, B. & Bendelac, A. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 18, 514–525 (2018).
Cohen, N. R., Garg, S. & Brenner, M. B. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv. Immunol. 102, 1–94 (2009).
Nielsen, M. M., Witherden, D. A. & Havran, W. L. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 17, 733–745 (2017).
Dadi, S. et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 164, 365–377 (2016).
Chou, C. et al. Programme of self-reactive innate-like T cell-mediated cancer immunity. Nature https://doi.org/10.1038/s41586-022-04632-1 (2022). The studies by Dadi et al. (2016) and Chou et al. (2022) demonstrate that induction of tissue-resident phenotype killer innate lymphocytes and innate-like T cells defines a discrete prewired cancer surveillance response and that TCRαβ+ ILTCKs are a distinct lineage of self-reactive T cells.
Konkel, J. E. et al. Control of the development of CD8alphaalpha+ intestinal intraepithelial lymphocytes by TGF-beta. Nat. Immunol. 12, 312–319 (2011).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
Wu, Y. et al. An innate-like Vdelta1+ gammadelta T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aax9364 (2019).
Boufea, K. et al. Single-cell RNA sequencing of human breast tumour-infiltrating immune cells reveals a gammadelta T-cell subtype associated with good clinical outcome. Life Sci. Alliance https://doi.org/10.26508/lsa.202000680 (2021).
Peters, C. et al. TGF-beta enhances the cytotoxic activity of Vdelta2 T cells. Oncoimmunology 8, e1522471 (2019).
Doisne, J. M. et al. iNKT cell development is orchestrated by different branches of TGF-beta signaling. J. Exp. Med. 206, 1365–1378 (2009).
Havenar-Daughton, C., Li, S., Benlagha, K. & Marie, J. C. Development and function of murine RORgammat+ iNKT cells are under TGF-beta signaling control. Blood 119, 3486–3494 (2012).
Moodycliffe, A. M., Nghiem, D., Clydesdale, G. & Ullrich, S. E. Immune suppression and skin cancer development: regulation by NKT cells. Nat. Immunol. 1, 521–525 (2000).
Terabe, M. et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J. Exp. Med. 202, 1627–1633 (2005).
Kobayashi, E., Motoki, K., Uchida, T., Fukushima, H. & Koezuka, Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol. Res. 7, 529–534 (1995).
Toura, I. et al. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J. Immunol. 163, 2387–2391 (1999).
Do, J. S. et al. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J. Immunol. 184, 1675–1679 (2010).
Coffelt, S. B. et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Borkowski, T. A., Letterio, J. J., Farr, A. G. & Udey, M. C. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 184, 2417–2422 (1996).
Dalessandri, T., Crawford, G., Hayes, M., Castro Seoane, R. & Strid, J. IL-13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat. Commun. 7, 12080 (2016).
Crawford, G. et al. Epithelial damage and tissue gammadelta T cells promote a unique tumor-protective IgE response. Nat. Immunol. 19, 859–870 (2018).
Cazac, B. B. & Roes, J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13, 443–451 (2000).
Reboldi, A. et al. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352, aaf4822 (2016).
Wallace, C. H. et al. B lymphocytes confer immune tolerance via cell surface GARP-TGF-beta complex. JCI Insight https://doi.org/10.1172/jci.insight.99863 (2018).
Albright, A. R. et al. TGFbeta signaling in germinal center B cells promotes the transition from light zone to dark zone. J. Exp. Med. 216, 2531–2545 (2019). This study uncovers discrete types of TGFβ signalling that influence B cell biology, with signalling in the Peyer patches inducing IgA class-switching in activated B cells, and signalling in the germinal centre required for transition from the light zone to the dark zone.
Hollern, D. P. et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell 179, 1191–1206 e1121 (2019).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Shalapour, S. et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98 (2015).
Viel, S. et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 9, ra19 (2016). This study reveals that TGFβ suppresses activation, cytolytic activity and cancer metastasis surveillance function of NK cells in association with TGFβ control of mTOR complex1 signalling.
Gao, Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 18, 1004–1015 (2017).
Cortez, V. S. et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat. Immunol. 18, 995–1003 (2017).
Zaiatz-Bittencourt, V., Finlay, D. K. & Gardiner, C. M. Canonical TGF-beta signaling pathway represses human NK cell metabolism. J. Immunol. 200, 3934–3941 (2018).
Nixon, B. G. et al. Cytotoxic granzyme C-expressing ILC1s contribute to antitumor immunity and neonatal autoimmunity. Sci. Immunol. 7, eabi8642 (2022). This study demonstrates that ILC1s can exhibit lytic granule-mediated cytotoxicity and that TGFβ signalling is required to maintain the antitumour function of CD103+ ILC1s in a transgenic model of breast cancer.
Kansler, E. R. et al. Cytotoxic innate lymphoid cells sense cancer cell-expressed interleukin-15 to suppress human and murine malignancies. Nat. Immunol. 23, 904–915 (2022).
Moreno-Nieves, U. Y. et al. Landscape of innate lymphoid cells in human head and neck cancer reveals divergent NK cell states in the tumor microenvironment. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2101169118 (2021).
Wang, L. et al. TGF-beta induces ST2 and programs ILC2 development. Nat. Commun. 11, 35 (2020).
Moral, J. A. et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 579, 130–135 (2020).
Jacquelot, N. et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 22, 851–864 (2021).
Wang, S. et al. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 30, 610–622 (2020).
Murphy, T. L. et al. Transcriptional control of dendritic cell development. Annu. Rev. Immunol. 34, 93–119 (2016).
Ramalingam, R. et al. Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J. Immunol. 189, 3878–3893 (2012).
Bain, C. C. et al. TGFbetaR signalling controls CD103+CD11b+ dendritic cell development in the intestine. Nat. Commun. 8, 620 (2017).
Kaplan, D. H. et al. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J. Exp. Med. 204, 2545–2552 (2007).
Yu, X. et al. The cytokine TGF-beta promotes the development and homeostasis of alveolar macrophages. Immunity 47, 903–912 e904 (2017).
Modi, B. G. et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science 335, 104–108 (2012).
Ortner, D. et al. Langerhans cells and NK cells cooperate in the inhibition of chemical skin carcinogenesis. Oncoimmunology 6, e1260215 (2017).
Franklin, R. A. et al. The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925 (2014).
Wahl, S. M. et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc. Natl Acad. Sci. USA 84, 5788–5792 (1987).
Sun, X., Robertson, S. A. & Ingman, W. V. Regulation of epithelial cell turnover and macrophage phenotype by epithelial cell-derived transforming growth factor beta1 in the mammary gland. Cytokine 61, 377–388 (2013).
Rani, R., Smulian, A. G., Greaves, D. R., Hogan, S. P. & Herbert, D. R. TGF-beta limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur. J. Immunol. 41, 2000–2009 (2011).
Ryzhov, S. V. et al. Role of TGF-beta signaling in generation of CD39+CD73+ myeloid cells in tumors. J. Immunol. 193, 3155–3164 (2014).
Novitskiy, S. V. et al. Deletion of TGF-beta signaling in myeloid cells enhances their anti-tumorigenic properties. J. Leukoc. Biol. 92, 641–651 (2012).
Pang, Y. et al. TGF-beta signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 3, 936–951 (2013).
Meng, X. et al. Myeloid-specific TGF-beta signaling in bone promotes basic-FGF and breast cancer bone metastasis. Oncogene 35, 2370–2378 (2016).
Sun, X. et al. Attenuated TGFB signalling in macrophages decreases susceptibility to DMBA-induced mammary cancer in mice. Breast Cancer Res. 23, 39 (2021).
Guerin, M. V. et al. TGFbeta blocks IFNalpha/beta release and tumor rejection in spontaneous mammary tumors. Nat. Commun. 10, 4131 (2019). This study reveals differences in macrophage responsiveness to STING activation in transplantable versus spontaneous tumour models, showing spontaneous tumours to be higher in TGFβ, which limits the interferon-induced tumour regression.
Xu, P. et al. Innate antiviral host defense attenuates TGF-beta function through IRF3-mediated suppression of Smad signaling. Mol. Cell 56, 723–737 (2014).
Hedrick, C. C. & Malanchi, I. Neutrophils in cancer: heterogeneous and multifaceted. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-021-00571-6 (2021).
Jackstadt, R. et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell 36, 319–336 e317 (2019).
Brandes, M. E., Mai, U. E., Ohura, K. & Wahl, S. M. Type I transforming growth factor-beta receptors on neutrophils mediate chemotaxis to transforming growth factor-beta. J. Immunol. 147, 1600–1606 (1991).
Fava, R. A. et al. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J. Exp. Med. 173, 1121–1132 (1991).
Yang, Y. et al. The outcome of TGFbeta antagonism in metastatic breast cancer models in vivo reflects a complex balance between tumor-suppressive and proprogression activities of TGFbeta. Clin. Cancer Res. 26, 643–656 (2020).
Novitskiy, S. V. et al. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 1, 430–441 (2011).
Dhainaut, M. et al. Spatial CRISPR genomics identifies regulators of the tumor microenvironment. Cell 185, 1223–1239 e1220 (2022).
Chakravarthy, A., Khan, L., Bensler, N. P., Bose, P. & De Carvalho, D. D. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9, 4692 (2018).
Mariathasan, S. et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Tauriello, D. V. F. et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018). The studies by Mariathasan et al. (2018) and Tauriello et al. (2018) demonstrate that increased TGFβ levels in tumour tissue are associated with T cell exclusion in patients with metastatic cancer and that blockade of TGFβ signalling synergizes with anti-PDL1 to promote antitumour T cell immunity in transplantable primary and metastatic tumour models.
Grauel, A. L. et al. TGFbeta-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat. Commun. 11, 6315 (2020).
Bhowmick, N. A. et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851 (2004).
Achyut, B. R. et al. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-beta signaling. PLoS Genet. 9, e1003251 (2013).
Anderton, M. J. et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol. Pathol. 39, 916–924 (2011).
Conroy, M. & Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 13, 392 (2022).
Martin, C. J. et al. Selective inhibition of TGFbeta1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aay8456 (2020).
Oida, T. & Weiner, H. L. TGF-beta induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS ONE 5, e15523 (2010).
Gabriely, G. et al. Targeting latency-associated peptide promotes antitumor immunity. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aaj1738 (2017).
Bertrand, C. et al. Combined blockade of GARP:TGF-beta1 and PD-1 increases infiltration of T cells and density of pericyte-covered GARP+ blood vessels in mouse MC38 tumors. Front. Immunol. 12, 704050 (2021).
Hezel, A. F. et al. TGF-beta and alphavbeta6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res. 72, 4840–4845 (2012).
Reader, C. S. et al. The integrin alphavbeta6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J. Pathol. 249, 332–342 (2019).
Vanpouille-Box, C. et al. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 75, 2232–2242 (2015).
Rodriguez-Ruiz, M. E. et al. TGFbeta blockade enhances radiotherapy abscopal efficacy effects in combination with anti-PD1 and anti-CD137 immunostimulatory monoclonal antibodies. Mol. Cancer Ther. 18, 621–631 (2019).
Formenti, S. C. et al. Focal irradiation and systemic TGFbeta blockade in metastatic breast cancer. Clin. Cancer Res. 24, 2493–2504 (2018).
Greco, R. et al. Pan-TGFbeta inhibition by SAR439459 relieves immunosuppression and improves antitumor efficacy of PD-1 blockade. Oncoimmunology 9, 1811605 (2020).
Terabe, M. et al. Blockade of only TGF-beta 1 and 2 is sufficient to enhance the efficacy of vaccine and PD-1 checkpoint blockade immunotherapy. Oncoimmunology 6, e1308616 (2017).
Nizard, M. et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 8, 15221 (2017).
Lin, H. Y. et al. The soluble exoplasmic domain of the type II transforming growth factor (TGF)-beta receptor. A heterogeneously glycosylated protein with high affinity and selectivity for TGF-beta ligands. J. Biol. Chem. 270, 2747–2754 (1995).
Lan, Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci. Transl. Med. 10, https://doi.org/10.1126/scitranslmed.aan5488 (2018). This study reveals that the bifunctional molecule M7824, with fusion of the extracellular domain of TGFBR2 (TGFβ trap) to a PDL1 antibody, suppresses primary tumour growth and metastasis more effectively than anti-PDL1 or TGFβ trap alone in transplantable tumour models.
Strauss, J. et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFbeta, in advanced solid tumors. Clin. Cancer Res. 24, 1287–1295 (2018).
Ravi, R. et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat. Commun. 9, 741 (2018).
Yingling, J. M. et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-beta receptor type I inhibitor. Oncotarget 9, 6659–6677 (2018).
Holmgaard, R. B. et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J. Immunother. Cancer 6, 47 (2018).
Kelley, R. K. et al. A phase 2 study of galunisertib (TGF-beta1 receptor type I inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clin. Transl. Gastroenterol. 10, e00056 (2019).
Ikeda, M. et al. A phase 1b study of transforming growth factor-beta receptor I inhibitor galunisertib in combination with sorafenib in Japanese patients with unresectable hepatocellular carcinoma. Invest. N. Drugs 37, 118–126 (2019).
Harding, J. J. et al. Phase 1b study of galunisertib and ramucirumab in patients with advanced hepatocellular carcinoma. Cancer Med. 10, 3059–3067 (2021).
Melisi, D. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 119, 1208–1214 (2018).
Melisi, D. et al. Safety and activity of the TGFbeta receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-002068 (2021).
Li, S. et al. Cancer immunotherapy via targeted TGF-beta signalling blockade in TH cells. Nature 587, 121–125 (2020). This study shows that a CD4+ T cell-directed TGFβ inhibitor, 4T-Trap, with fusion of the extracellular domain of TGFBR2 (TGFβ trap) to a CD4 antibody, but not a non-targeted TGFβ trap, suppresses tumour growth in a transgenic model of breast cancer.
Yi, M. et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-beta and PD-L1. J. Hematol. Oncol. 14, 27 (2021).
Zhang, L. et al. Inhibition of TGF-beta signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther. 20, 575–580 (2013).
Bollard, C. M. et al. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed Hodgkin lymphoma. J. Clin. Oncol. 36, 1128–1139 (2018). This study shows that adoptive transfer of Epstein–Barr virus antigen-specific T cells overexpressing a dominant-negative mutant of TGFBR2 is safe and can induce complete responses even in patients with Hodgkin lymphoma with resistant disease.
Kloss, C. C. et al. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Tang, N. et al. TGF-beta inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight https://doi.org/10.1172/jci.insight.133977 (2020).
Chen, X. et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-beta trap enhances antitumor efficacy of CAR-T cell therapy. Mol. Ther. Oncolytics 21, 144–157 (2021).
Narayan, V. et al. PSMA-targeting TGFbeta-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. https://doi.org/10.1038/s41591-022-01726-1 (2022). This study shows that adoptive transfer of PSMA-targeting CAR T cells overexpressing a dominant-negative mutant of TGFBR2 is generally safe in patients with metastatic castration-resistant prostate cancer, with evidence of tumour regression being observed in one of the 13 patients.
Lucas, P. J. et al. Transforming growth factor-beta pathway serves as a primary tumor suppressor in CD8+ T cell tumorigenesis. Cancer Res. 64, 6524–6529 (2004).
Ishigame, H., Mosaheb, M. M., Sanjabi, S. & Flavell, R. A. Truncated form of TGF-betaRII, but not its absence, induces memory CD8+ T cell expansion and lymphoproliferative disorder in mice. J. Immunol. 190, 6340–6350 (2013).
Roth, T. L. et al. Pooled knockin targeting for genome engineering of cellular immunotherapies. Cell 181, 728–744 e721 (2020).
Chang, Z. L. et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 14, 317–324 (2018).
Burga, R. A. et al. Engineering the TGFbeta receptor to enhance the therapeutic potential of natural killer cells as an immunotherapy for neuroblastoma. Clin. Cancer Res. 25, 4400–4412 (2019).
Wang, Q. M. et al. Enhanced cancer immunotherapy with Smad3-silenced NK-92 cells. Cancer Immunol. Res. 6, 965–977 (2018).
Guerin, M. V., Finisguerra, V., Van den Eynde, B. J., Bercovici, N. & Trautmann, A. Preclinical murine tumor models: a structural and functional perspective. Elife 9, https://doi.org/10.7554/eLife.50740 (2020).
Acknowledgements
The authors apologize to those whose work they could not cite owing to space constraints. They thank former and current M.O.L. laboratory members for discussions. This work was supported by the US National Institutes of Health Memorial Sloan Kettering Cancer Center Core Grant (P30 CA08748).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
Memorial Sloan Kettering Cancer Center holds a patent entitled “Methods and compositions for targeting TGF-β signalling in CD4+ helper T cells for cancer immunotherapy” with M.O.L. listed as an inventor. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks R. Akhurst, S.-Y. Chen, P. ten Dijke, E. Jannessen, D. Tauriello and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Adaptive immunity
-
Immunity mediated by leukocytes that express an antigen-specific receptor (T cell receptor or B cell receptor) that drives both their development in primary lymphoid organs and their function in the periphery.
- Autochthonous tumour models
-
Tumour models where tumour initiation is by cells of an endogenous organ in an intact animal via processes such as oncogene expression or carcinogen exposure.
- Innate immunity
-
Immunity mediated by leukocytes that express germ line-encoded receptors that recognize common, broad patterns associated with pathogens and cell stress.
- Prewired immune response
-
Immune response involving innate myeloid cells as well as innate lymphocytes and innate-like adaptive lymphocytes that reside in premalignant tissues or migrate to tumours directly after development in primary lymphoid organs.
- Priming-dependent immune response
-
Immune response involving adaptive lymphocytes that are primed in secondary lymphoid organs before homing to tumours.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nixon, B.G., Gao, S., Wang, X. et al. TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective. Nat Rev Immunol 23, 346–362 (2023). https://doi.org/10.1038/s41577-022-00796-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-022-00796-z
- Springer Nature Limited
This article is cited by
-
First-in-human study of GFH018, a small molecule inhibitor of transforming growth factor-β receptor I inhibitor, in patients with advanced solid tumors
BMC Cancer (2024)
-
Functional CRISPR screens in T cells reveal new opportunities for cancer immunotherapies
Molecular Cancer (2024)
-
Regulatory T cell-mediated immunosuppression orchestrated by cancer: towards an immuno-genomic paradigm for precision medicine
Nature Reviews Clinical Oncology (2024)
-
Single-cell sequencing reveals VEGFR as a potential target for CAR-T cell therapy in chordoma
British Journal of Cancer (2024)
-
The immune regulation and therapeutic potential of the SMAD gene family in breast cancer
Scientific Reports (2024)