Abstract
During the past 5 years, a number of highly active novel agents, including kinase inhibitors targeting BTK or PI3Kδ, an antagonist of the antiapoptotic protein BCL-2, and new anti-CD20 monoclonal antibodies, have been added to the therapeutic armamentarium for patients with chronic lymphocytic leukaemia (CLL). In these exciting times, care is needed to optimally integrate these novel agents into the traditional treatment algorithm without overlooking or compromising the benefits of established treatments, especially chemoimmunotherapy. A more personalized approach to CLL therapy that takes into account individual risk factors, patient characteristics, and their treatment preferences is now possible. Herein, we discuss the biological basis for the novel therapeutic agents and outline not only the major advantages of these agents over traditional therapies but also their adverse effects and the rationale for continued use of older versus newer types of therapy for selected patients with CLL. We conclude by providing recommendations for an individualized therapy approach for different populations of patients with CLL.
Key points
Novel drugs enable targeting of key pathogenic pathways of chronic lymphocytic leukaemia (CLL), including BTK, SYK, and PI3Kδ inhibitors that disrupt B cell receptor signalling and venetoclax, an antagonist of the anti-apoptotic protein BCL-2.
The kinase inhibitors typically cause mobilization of tissue-resident CLL cells into the circulation, resulting in rapid resolution of lymphadenopathy and splenomegaly and transient redistribution lymphocytosis.
Kinase inhibitor monotherapy mostly induces partial remissions, which are typically maintained with long-term therapy but can be compromised by toxicities and the emergence of resistance; hence, limited-duration therapy, if effective, would be desirable.
Venetoclax has direct cytotoxic activity, sometimes resulting in tumour lysis; this drug has mostly been used after failure of kinase inhibitor therapy, but emerging data support its use in earlier treatment lines and/or in combination with other agents.
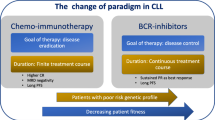
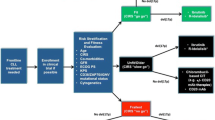
Given the expanding therapeutic armamentarium for CLL, risk-based individualized therapy is now possible, ranging from chemoimmunotherapy for low-risk (IGHV-mutated) CLL to novel molecularly targeted therapy — primarily with BTK inhibitors — for high-risk (del(17p), IGHV-unmutated) disease.
New anti-CD20 antibodies, immunomodulatory agents, and cellular therapies (such as chimeric antigen receptor T cells and allogeneic haematopoietic stem cell transplantation) are additional important components of CLL treatment.





Similar content being viewed by others
References
Kipps, T. J. et al. Chronic lymphocytic leukaemia. Nat. Rev. Dis. Primers 3, 16096 (2017).
Dores, G. M. et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br. J. Haematol. 139, 809–819 (2007).
Chiorazzi, N., Rai, K. R. & Ferrarini, M. Chronic lymphocytic leukemia. N. Engl. J. Med. 352, 804–815 (2005).
Hallek, M. et al. Guidelines for diagnosis, indications for treatment, response assessment and supportive management of chronic lymphocytic leukemia. Blood https://doi.org/10.1182/blood-2017-09-806398 (2018).
Herndon, T. M. et al. Direct in vivo evidence for increased proliferation of CLL cells in lymph nodes compared to bone marrow and peripheral blood. Leukemia 31, 1340–1347 (2017).
Herishanu, Y. et al. The lymph node microenvironment promotes B cell receptor signaling, NF-κB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 117, 563–574 (2011).
Rai, K. R. et al. Clinical staging of chronic lymphocytic leukemia. Blood 46, 219–234 (1975).
Binet, J. L. et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 48, 198–206 (1981).
Dohner, H. et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 343, 1910–1916 (2000).
Fais, F. et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J. Clin. Invest. 102, 1515–1525 (1998).
Hashimoto, S. et al. Somatic diversification and selection of immunoglobulin heavy and light chain variable region genes in IgG+ CD5+ chronic lymphocytic leukemia B cells. J. Exp. Med. 181, 1507–1517 (1995).
Damle, R. N. et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 94, 1840–1847 (1999).
Hamblin, T. J., Davis, Z., Gardiner, A., Oscier, D. G. & Stevenson, F. K. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 94, 1848–1854 (1999).
The International CLL-IPI Working Group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 17, 779–790 (2016).
Thompson, P. A. et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 127, 303–309 (2016).
Stilgenbauer, S. et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood 123, 3247–3254 (2014).
Keating, M. J. et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J. Clin. Oncol. 23, 4079–4088 (2005).
Hallek, M. et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376, 1164–1174 (2010).
Fischer, K. et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J. Clin. Oncol. 29, 3559–3566 (2011).
Eichhorst, B. et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 17, 928–942 (2016).
Thompson, P. A. & Wierda, W. G. Eliminating minimal residual disease as a therapeutic end point: working toward cure for patients with CLL. Blood 127, 279–286 (2016).
Rai, K. R. et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N. Engl. J. Med. 343, 1750–1757 (2000).
O’Brien, S. M. et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J. Clin. Oncol. 19, 2165–2170 (2001).
Goede, V. et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med. 370, 1101–1110 (2014).
Byrd, J. C. et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 369, 32–42 (2013).
Burger, J. A. et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 373, 2425–2437 (2015).
Furman, R. R. et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 370, 997–1007 (2014).
Roberts, A. W. et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2016).
Farooqui, M. Z. et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 16, 169–176 (2015).
Stilgenbauer, S. et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 17, 768–778 (2016).
Hillmen, P. et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet 385, 1873–1883 (2015).
Pearson, O. H. et al. Adrenocorticotropic hormone- and cortisone-induced regression of lymphoid tumors in man; a preliminary report. Cancer 2, 943–945 (1949).
Pearson, O. H. & Eliel, L. P. Use of pituitary adrenocorticotropic hormone (ACTH) and cortisone in lymphomas and leukemias. J. Am. Med. Assoc. 144, 1349–1353 (1950).
Rosenthal, M. C. et al. The use of adrenocorticotropic hormone and cortisone in the treatment of leukemia and leukosarcoma. Blood 6, 804–823 (1951).
Freymann, J. G., Vander, J. B., Marler, E. A. & Meyer, D. G. Prolonged corticosteroid therapy of chronic lymphocytic leukaemia and the closely allied malignant lymphomas. Br. J. Haematol. 6, 303–323 (1960).
Galton, D. A., Wiltshaw, E., Szur, L. & Dacie, J. V. The use of chlorambucil and steroids in the treatment of chronic lymphocytic leukaemia. Br. J. Haematol. 7, 73–98 (1961).
Shaw, R. K., Boggs, D. R., Silberman, H. R. & Frei, E. 3rd A study of prednisone therapy in chronic lymphocytic leukemia. Blood 17, 182–195 (1961).
Dighiero, G., Vaugier, G., Charron, D., d’Athis, P. & Binet, J. L. Variations in lymphocyte counts four hours after administration of hydrocortisone in patients with chronic lymphocytic leukemia. Blood 49, 719–728 (1977).
Burger, J. A. & Montserrat, E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B cell receptor signaling. Blood 121, 1501–1509 (2013).
Thornton, P. D. et al. High dose methylprednisolone can induce remissions in CLL patients with p53 abnormalities. Ann. Hematol. 82, 759–765 (2003).
Pettitt, A. R. et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia and deletion of TP53: final results of the national cancer research institute CLL206 trial. J. Clin. Oncol. 30, 1647–1655 (2012).
Castro, J. E. et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia 23, 1779–1789 (2009).
Knospe, W. H., Loeb, V. Jr & Huguley, C. M. Jr. Proceedings: bi-weekly chlorambucil treatment of chronic lymphocytic leukemia. Cancer 33, 555–562 (1974).
Catovsky, D., Else, M. & Richards, S. Chlorambucil — still not bad: a reappraisal. Clin. Lymphoma Myeloma Leuk. 11(Suppl. 1), S2–S6 (2011).
Dighiero, G. et al. Chlorambucil in indolent chronic lymphocytic leukemia. N. Engl. J. Med. 338, 1506–1514 (1998).
Keating, M. J. et al. Fludarabine: a new agent with major activity against chronic lymphocytic leukemia. Blood 74, 19–25 (1989).
Eichhorst, B. F. et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 114, 3382–3391 (2009).
Knauf, W. U. et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J. Clin. Oncol. 27, 4378–4384 (2009).
Fischer, K. et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J. Clin. Oncol. 30, 3209–3216 (2012).
Keating, M. J. et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood 92, 1165–1171 (1998).
The French Cooperative Group on CLL et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia. Lancet 347, 1432–1438 (1996).
O’Brien, S. M. et al. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J. Clin. Oncol. 19, 1414–1420 (2001).
Eichhorst, B. F. et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 107, 885–891 (2006).
Robak, T. et al. Cladribine with or without prednisone in the treatment of previously treated and untreated B cell chronic lymphocytic leukaemia — updated results of the multicentre study of 378 patients. Br. J. Haematol. 108, 357–368 (2000).
Robak, T. et al. Cladribine with prednisone versus chlorambucil with prednisone as first-line therapy in chronic lymphocytic leukemia: report of a prospective, randomized, multicenter trial. Blood 96, 2723–2729 (2000).
Kay, N. E. et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood 109, 405–411 (2007).
Kay, N. E. et al. Cumulative experience and long term follow-up of pentostatin-based chemoimmunotherapy trials for patients with chronic lymphocytic leukemia. Expert Rev. Hematol. 11, 337–349 (2018).
Lamanna, N. et al. Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J. Clin. Oncol. 24, 1575–1581 (2006).
Wierda, W. et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J. Clin. Oncol. 23, 4070–4078 (2005).
Fischer, K. et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 127, 208–215 (2016).
Strati, P. et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood 123, 3727–3732 (2014).
Benjamini, O. et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk. Lymphoma 56, 1643–1650 (2015).
Friedberg, J. W. et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115, 2578–2585 (2010).
Advani, R. H. et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B cell malignancies. J. Clin. Oncol. 31, 88–94 (2013).
Gopal, A. K. et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 370, 1008–1018 (2014).
Chang, B. Y. et al. Egress of CD19(+)CD5(+) cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood 122, 2412–2424 (2013).
Spaargaren, M. et al. The B cell antigen receptor controls integrin activity through Btk and PLCγ2. J. Exp. Med. 198, 1539–1550 (2003).
de Gorter, D. J. et al. Bruton’s tyrosine kinase and phospholipase Cγ2 mediate chemokine-controlled B cell migration and homing. Immunity 26, 93–104 (2007).
Burger, J. A. & Buggy, J. J. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk. Lymphoma 54, 2385–2391 (2013).
Wang, M. L. et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 369, 507–516 (2013).
Treon, S. P. et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N. Engl. J. Med. 372, 1430–1440 (2015).
Noy, A. et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 129, 2224–2232 (2017).
Wilson, W. H. et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 21, 922–926 (2015).
O’Brien, S. et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood https://doi.org/10.1182/blood-2017-10-810044 (2018).
Woyach, J. A. et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood 123, 1810–1817 (2014).
Burger, J. A. et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 15, 1090–1099 (2014).
Chanan-Khan, A. et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 17, 200–211 (2016).
Jain, N. et al. Combined venetoclax and ibrutinib for patients with previously untreated high-risk CLL, and relapsed/refractory CLL: a phase II trial. Blood 130, 429–429 (2017).
Byrd, J. C. et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 371, 213–223 (2014).
Mato, A. R. et al. Toxicities and outcomes of 621 ibrutinib-treated chronic lymphocytic leukemia patients in the United States: a real-world analysis. Haematologica https://doi.org/10.3324/haematol.2017.182907 (2018).
Maddocks, K. J. et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 1, 80–87 (2015).
Thompson, P. A. et al. Complex karyotype is a stronger predictor than del(17p) for an inferior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib-based regimens. Cancer 121, 3612–3621 (2015).
Burger, J. A. et al. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat. Commun. 7, 11589 (2016).
Woyach, J. A. et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 370, 2286–2294 (2014).
Woyach, J. A. et al. BTKC481S-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J. Clin. Oncol. 35, 1437–1443 (2017).
Ahn, I. E. et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood 129, 1469–1479 (2017).
Hoellenriegel, J. et al. The phosphoinositide 3ʹ -kinase delta inhibitor, CAL-101, inhibits B cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 118, 3603–3612 (2011).
Lannutti, B. J. et al. CAL-101, a p110δ selective phosphatidylinositol-3-kinase inhibitor for the treatment of B cell malignancies, inhibits PI3K signaling and cellular viability. Blood 117, 591–594 (2011).
Brown, J. R. et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 123, 3390–3397 (2014).
Flinn, I. W. et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood 123, 3406–3413 (2014).
Lampson, B. L. et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood 128, 195–203 (2016).
O’Brien, S. M. et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood 126, 2686–2694 (2015).
Zelenetz, A. D. et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 18, 297–311 (2017).
Smith, S. M. et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol. 4, e176–e182 (2017).
Jones, J. A. et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 4, e114–e126 (2017).
Ali, K. et al. Inactivation of PI(3)K p110δ breaks regulatory T cell-mediated immune tolerance to cancer. Nature 510, 407–411 (2014).
Byrd, J. C. et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 323–332 (2016).
Honigberg, L. A. et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B cell activation and is efficacious in models of autoimmune disease and B cell malignancy. Proc. Natl Acad. Sci. USA 107, 13075–13080 (2010).
Walter, H. S. et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B cell malignancies. Blood 127, 411–419 (2016).
Tam, C. S. et al. Twice daily dosing with the highly specific BTK inhibitor, Bgb-3111, achieves complete and continuous BTK occupancy in lymph nodes, and is associated with durable responses in patients (pts) with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Blood 128, 642–642 (2016).
Neuman, L. L. et al. First-in-human phase 1a study of the safety, pharmacokinetics, and pharmacodynamics of the noncovalent bruton tyrosine kinase (BTK) inhibitor SNS-062 in healthy subjects. Blood 128, 2032–2032 (2016).
Flinn, I. W. et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood 131, 877–887 (2018).
Flinn, I. W. et al. Combination trial of duvelisib (IPI-145) with bendamustine, rituximab, or bendamustine/rituximab in patients with lymphoma or chronic lymphocytic leukemia. Blood 126, 3928–3928 (2015).
Davids, M. S. et al. Preliminary results of a phase Ib study of duvelisib in combination with FCR (dFCR) in previously untreated, younger patients with CLL. Blood 126, 4158–4158 (2015).
Burris, H.A. 3rd et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 19, 486–496 (2018).
Braselmann, S. et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 319, 998–1008 (2006).
Weinblatt, M. E. et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 363, 1303–1312 (2010).
Currie, K. S. et al. Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J. Med. Chem. 57, 3856–3873 (2014).
Sharman, J. et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood 125, 2336–2343 (2015).
Contri, A. et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J. Clin. Invest. 115, 369–378 (2005).
Amrein, P. C. et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin. Cancer Res. 17, 2977–2986 (2011).
Ma, S. et al. Deep and durable responses following venetoclax (ABT-199 / GDC-0199) combined with rituximab in patients with relapsed/refractory chronic lymphocytic leukemia: results from a phase 1b study. Blood 126, 830 (2015).
Seymour, J. F. et al. Venetoclax–rituximab in relapsed or refractory chronic lymphocytic leukemia. N. Engl. J. Med. 378, 1107–1120 (2018).
Jones, J. A. et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 19, 65–75 (2018).
Teeling, J. L. et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J. Immunol. 177, 362–371 (2006).
Pawluczkowycz, A. W. et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J. Immunol. 183, 749–758 (2009).
Wierda, W. G. et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 28, 1749–1755 (2010).
Mossner, E. et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B cell cytotoxicity. Blood 115, 4393–4402 (2010).
Cartron, G. et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood 124, 2196–2202 (2014).
de Romeuf, C. et al. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcgammaRIIIA/CD16. Br. J. Haematol. 140, 635–643 (2008).
Sawas, A. et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br. J. Haematol. 177, 243–253 (2017).
Woyach, J. A. et al. A phase 1 trial of the Fc-ngineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood 124, 3553–3560 (2014).
Byrd, J. C. et al. A phase 1 study evaluating the safety and tolerability of otlertuzumab, an anti-CD37 mono-specific ADAPTIR therapeutic protein in chronic lymphocytic leukemia. Blood 123, 1302–1308 (2014).
Robak, T. et al. Randomized phase 2 study of otlertuzumab and bendamustine versus bendamustine in patients with relapsed chronic lymphocytic leukaemia. Br. J. Haematol. 176, 618–628 (2017).
Ramsay, A. G. et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J. Clin. Invest. 118, 2427–2437 (2008).
Fecteau, J. F. et al. Lenalidomide inhibits the proliferation of CLL cells via a cereblon/p21(WAF1/Cip1)-dependent mechanism independent of functional p53. Blood 124, 1637–1644 (2014).
Takahashi, K. et al. Clinical implications of cancer gene mutations in patients with chronic lymphocytic leukemia treated with lenalidomide. Blood 131, 1820–1832 (2018).
Badoux, X. C. et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood 118, 3489–3498 (2011).
Ferrajoli, A. et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood 111, 5291–5297 (2008).
Badoux, X. C. et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. 31, 584–591 (2013).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl Med. 5, 177ra38 (2013).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl Med. 7, 303ra139 (2015).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Turtle, C. J. et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J. Clin. Oncol. 35, 3010–3020 (2017).
Brown, J. R. et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia 32, 83–91 (2018).
Jain, P. et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood 125, 2062–2067 (2015).
Dreger, P. et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood 124, 3841–3849 (2014).
Sutton, L. et al. Autologous stem cell transplantation as a first-line treatment strategy for chronic lymphocytic leukemia: a multicenter, randomized, controlled trial from the SFGM-TC and GFLLC. Blood 117, 6109–6119 (2011).
Kramer, I. et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood 130, 1477–1480 (2017).
Tsimberidou, A. M. et al. Phase I-II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter’s syndrome or fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 26, 196–203 (2008).
Ding, W. et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 129, 3419–3427 (2017).
Jain, N. et al. Ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (GA101) (iFCG) for first-line treatment of patients with CLL with mutated IGHV and without TP53 aberrations [abstract]. Blood 130 (Suppl. 1), 495 (2017).
Landau, D. A. et al. The evolutionary landscape of chronic lymphocytic leukemia treated with ibrutinib targeted therapy. Nat. Commun. 8, 2185 (2017).
Mato, A. R. et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann. Oncol. 28, 1050–1056 (2017).
Burger, J. A., Ghia, P., Rosenwald, A. & Caligaris-Cappio, F. The microenvironment in mature B cell malignancies: a target for new treatment strategies. Blood 114, 3367–3375 (2009).
Hoogeboom, R. et al. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J. Exp. Med. 210, 59–70 (2013).
Burger, J. A. & Chiorazzi, N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 34, 592–601 (2013).
Hacken, E. T. et al. Calreticulin as a novel B cell receptor antigen in chronic lymphocytic leukemia. Haematologica 102, e394–e396 (2017).
Binder, M. et al. CLL B cell receptors can recognize themselves: alternative epitopes and structural clues for autostimulatory mechanisms in CLL. Blood 121, 239–241 (2013).
Duhren-von Minden, M. et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 489, 309–312 (2012).
Minici, C. et al. Distinct homotypic B cell receptor interactions shape the outcome of chronic lymphocytic leukaemia. Nat. Commun. 8, 15746 (2017).
Burger, J. A. et al. High-level expression of the T cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood 113, 3050–3058 (2009).
Hartmann, E. M., Rudelius, M., Burger, J. A. & Rosenwald, A. CCL3 chemokine expression by chronic lymphocytic leukemia cells orchestrates the composition of the microenvironment in lymph node infiltrates. Leuk. Lymphoma 57, 563–571 (2016).
Fiorcari, S. et al. Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia. Oncotarget 7, 65968–65981 (2016).
Nguyen, P. H. et al. LYN kinase in the tumor microenvironment is essential for the progression of chronic lymphocytic leukemia. Cancer Cell 30, 610–622 (2016).
Niu, H., Ye, B. H. & Dalla-Favera, R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 12, 1953–1961 (1998).
Ten Hacken, E. et al. Functional differences between IgM and IgD signaling in chronic lymphocytic leukemia. J. Immunol. 197, 2522–2531 (2016).
Burger, J. A. et al. Leukemia cell proliferation and death in chronic lymphocytic leukemia patients on therapy with the BTK inhibitor ibrutinib. JCI Insight 2, e89904 (2017).
Dameshek, W. Chronic lymphocytic leukemia — an accumulative disease of immunolgically incompetent lymphocytes. Blood 29, 566–584 (1967).
Wilson, J. D. & Nossal, G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet 2, 788–791 (1971).
Salsano, F., Froland, S. S., Natvig, J. B. & Michaelsen, T. E. Same idiotype of B-lymphocyte membrane IgD and IgM. Formal evidence for monoclonality of chronic lymphocytic leukemia cells. Scand. J. Immunol. 3, 841–846 (1974).
Caligaris-Cappio, F., Gobbi, M., Bofill, M. & Janossy, G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J. Exp. Med. 155, 623–628 (1982).
Matutes, E. et al. The immunological profile of B cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia 8, 1640–1645 (1994).
Kitada, S. et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood 91, 3379–3389 (1998).
Herman, S. E. et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 117, 6287–6296 (2011).
Ponader, S. et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 119, 1182–1189 (2012).
Badoux, X. C. et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood 117, 3016–3024 (2011).
Byrd, J. C. et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 125, 2497–2506 (2015).
Coutre, S. E. et al. Extended treatment with single-agent ibrutinib at the 420 mg dose leads to durable responses in chronic lymphocytic leukemia/small lymphocytic lymphoma. Clin. Cancer Res. 23, 1149–1155 (2017).
Byrd, J. C. et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated results from the phase 1/2 ACE-CL-001 study. Blood 130, 498–498 (2017).
Walter, H. S. et al. Long-term follow-up of patients with CLL treated with the selective Bruton’s tyrosine kinase inhibitor ONO/GS-4059. Blood 129, 2808–2810 (2017).
Seymour, J. F. et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 18, 230–240 (2017).
Strati, P. et al. Lenalidomide induces long-lasting responses in elderly patients with chronic lymphocytic leukemia. Blood 122, 734–737 (2013).
Byrd, J. C. et al. Rituximab using a thrice weekly dosing schedule in B cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J. Clin. Oncol. 19, 2153–2164 (2001).
Acknowledgements
The work of J.A.B. is supported by a Leukemia & Lymphoma Society Scholar Award in Clinical Research, the University of Texas MD Anderson Cancer Center Chronic Lymphocytic Leukemia (CLL) Moon Shot programme, the CLL Global Research Foundation, and in part by the MD Anderson Cancer Center Support Grant CA016672.
Reviewer information
Nature Reviews Clinical Oncology thanks M. Hallek, T. Robak, and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J.A.B. and S.O’B. contributed equally to all stages of preparation of this manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
J.A.B. has received research support from Gilead and Pharmacyclics. S.O’B. has received research support from Acerta, Gilead, Kite, Pharmacyclics, and TG Therapeutics.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov database: https://clinicaltrials.gov/
Rights and permissions
About this article
Cite this article
Burger, J.A., O’Brien, S. Evolution of CLL treatment — from chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol 15, 510–527 (2018). https://doi.org/10.1038/s41571-018-0037-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-018-0037-8
- Springer Nature Limited
This article is cited by
-
Study of the intestinal microbiota composition and the effect of treatment with intensive chemotherapy in patients recovered from acute leukemia
Scientific Reports (2024)
-
Zanubrutinib: past, present, and future
Blood Cancer Journal (2023)
-
Mechanisms of ferroptosis and targeted therapeutic approaches in lymphoma
Cell Death & Disease (2023)
-
Ibrutinib sensitizes CLL cells to venetoclax by interrupting TLR9-induced CD40 upregulation and protein translation
Leukemia (2023)
-
Expert consensus on the management of chronic lymphocytic leukaemia in Asia
Clinical and Experimental Medicine (2023)