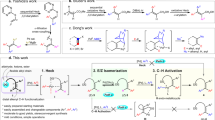
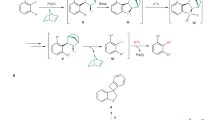
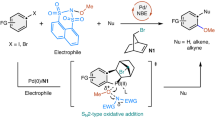
Abstract
Electrophilic aromatic substitution is among the most widely used mechanistic manifolds in organic chemistry. Access to certain substitution patterns is, however, precluded by intrinsic and immutable substituent effects that ultimately restrict the diversity of the benzenoid chemical space. Here we demonstrate that the established regioselectivity of electrophilic aromatic substitution can be overcome simply by diverting the key σ-complex intermediate towards otherwise inaccessible substitution products. This ‘regiodiversion’ strategy is realized through the development of a general and concise method for the meta-selective C–H arylation of sterically congested phenols. Consisting of a Bi(V)-mediated electrophilic arylation and a subsequent aryl migration/rearomatization, our process is orthogonal to conventional C–H activation and cross-coupling approaches, and does not require prefunctionalization of the substrate. Mechanistically informed applications in synthesis showcase its utility as a versatile and enabling route to highly functionalized, contiguously substituted aromatic building blocks that defy synthesis via existing methods.





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Taylor, R. Electrophilic Aromatic Substitution (John Wiley & Sons, Inc., 1990).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Scott, K. A. et al. A structural analysis of the FDA Green Book-approved veterinary drugs and roles in human medicine. J. Med. Chem. 63, 15449–15482 (2020).
Mortier, J. Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds (John Wiley & Sons, Inc., 2016).
Pratihar, S. & Roy, S. Nucleophilicity and site selectivity of commonly used arenes and heteroarenes. J. Org. Chem. 75, 4957–4963 (2010).
Kromann, J. C., Jensen, J. H., Kruszyk, M., Jessing, M. & Jørgensen, M. Fast and accurate prediction of the regioselectivity of electrophilic aromatic substitution reactions. Chem. Sci. 9, 660–665 (2018).
Nilova, A., Campeau, L.-C., Sherer, E. C. & Stuart, D. R. Analysis of benzenoid substitution patterns in small molecule active pharmaceutical ingredients. J. Med. Chem. 63, 13389–13396 (2020).
Boström, J., Brown, D. G., Young, R. J. & Keserü, G. M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 17, 709–727 (2018).
Brown, D. G., Gagnon, M. M. & Boström, J. Understanding our love affair with p-chlorophenyl: present day implications from historical biases of reagent selection. J. Med. Chem. 58, 2390–2405 (2015).
Sun, Z., Fridrich, B., de Santi, A., Elangovan, S. & Barta, K. Bright side of lignin depolymerization: toward new platform chemicals. Chem. Rev. 118, 614–678 (2018).
Huang, Z. & Lumb, J.-P. Phenol-directed C–H functionalization. ACS Catal. 9, 521–555 (2018).
Jurrat, M., Maggi, L., Lewis, W. & Ball, L. T. Modular bismacycles for the selective C–H arylation of phenols and naphthols. Nat. Chem. 12, 260–269 (2020).
Barton, D. H. R. et al. Comparative arylation reactions with pentaphenylbismuth and with triphenylbismuth carbonate. J. Chem. Soc. Chem. Commun. 1980, 827–829 (1980).
Barton, D. H. R. et al. Pentavalent organobismuth reagents. Part 2. The phenylation of phenols. J. Chem. Soc. Perkin Trans. 1 1985, 2657–2665 (1985).
Barton, D. H. R., Finet, J.-P., Giannotti, C. & Halley, F. The chemistry of pentavalent organobismuth reagents. Part 7. The possible role of radical mechanisms in the phenylation process for bismuth(V), and related lead(IV), iodine(III), and antimony(V) reagents. J. Chem. Soc. Perkin Trans. 1 1987(241), 249 (1987).
Barton, D. H. R. et al. The chemistry of pentavalent organobismuth reagents: Part X. Studies on the phenylation and oxidation of phenols. Tetrahedron 43, 323–332 (1987).
Wang, Z. in Comprehensive Organic Name Reactions and Reagents (ed. Wang, Z.) 900–904 (John Wiley & Sons, Inc., 2010).
Magdziak, D., Meek, S. J. & Pettus, T. R. R. Cyclohexadienone ketals and quinols: four building blocks potentially useful for enantioselective synthesis. Chem. Rev. 104, 1383–1430 (2004).
Quideau, S., Pouységu, L. & Deffieux, D. Oxidative dearomatization of phenols: why, how and what for? Synlett 2008, 467–495 (2008).
Pouységu, L., Deffieux, D. & Quideau, S. Hypervalent iodine-mediated phenol dearomatization in natural product synthesis. Tetrahedron 66, 2235–2261 (2010).
Ding, Q., Ye, Y. & Fan, R. Recent advances in phenol dearomatization and its application in complex syntheses. Synthesis 45, 1–16 (2013).
Wu, W.-T., Zhang, L. & You, S.-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 45, 1570–1580 (2016).
Wirth, T. in Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis (ed. Wirth, T.) 185–208 (Springer, 2003).
Oguma, T. & Katsuki, T. Iron-catalyzed dioxygen-driven C–C bond formation: oxidative dearomatization of 2-naphthols with construction of a chiral quaternary stereocenter. J. Am. Chem. Soc. 134, 20017–20020 (2012).
Zhang, Y. et al. Catalytic asymmetric hydroxylative dearomatization of 2-naphthols: synthesis of lacinilene derivatives. Chem. Sci. 8, 6645–6649 (2017).
Yin, Q. et al. Organocatalytic asymmetric chlorinative dearomatization of naphthols. Chem. Sci. 6, 4179–4183 (2015).
Vershinin, V., Dyadyuk, A. & Pappo, D. Iron-catalyzed selective oxidative arylation of phenols and biphenols. Tetrahedron 73, 3660–3668 (2017).
Dyadyuk, A. et al. Direct synthesis of polyaryls by consecutive oxidative cross-coupling of phenols with arenes. Org. Lett. 18, 4324–4327 (2016).
Guérard, K. C., Sabot, C., Racicot, L. & Canesi, S. Oxidative Friedel−Crafts reaction and its application to the total syntheses of amaryllidaceae alkaloids. J. Org. Chem. 74, 2039–2045 (2009).
Kirste, A., Elsler, B., Schnakenburg, G. & Waldvogel, S. R. Efficient anodic and direct phenol–arene C,C cross-coupling: the benign role of water or methanol. J. Am. Chem. Soc. 134, 3571–3576 (2012).
Quideau, S., Pouységu, L., Ozanne, A. & Gagnepain, J. Oxidative dearomatization of phenols and anilines via λ3- and λ5-iodane-mediated phenylation and oxygenation. Molecules 10, 201–216 (2005).
Ozanne-Beaudenon, A. & Quideau, S. Regioselective hypervalent-iodine(III)-mediated dearomatizing phenylation of phenols through direct ligand coupling. Angew. Chem. Int. Ed. 44, 7065–7069 (2005).
Morgan, J. & Pinhey, J. T. Mechanism of arylation of nucleophiles by aryllead triacetates. Part 1. Exclusion of a pathway involving aryl free radicals. J. Chem. Soc. Perkin Trans 1993, 1673–1676 (1993).
Bell, H. C., Pinhey, J. T. & Sternhell, S. The chemistry of aryllead(IV) tricarboxylates. Reaction with phenols. Aust. J. Chem. 32, 1551–1560 (1979).
Wittig, G. & Clauß, K. Pentaphenyl-wismut. Justus Liebigs Ann. Chem. 578, 136–146 (1952).
Miller, B. Too many rearrangements of cyclohexadienones. Acc. Chem. Res. 8, 245–256 (1975).
Shubin, V. G. in Contemporary Problems in Carbonium Ion Chemistry I/II (ed. Rees, C.) 267–341 (Springer, 1984).
He, M. & Swager, T. M. Aryl migration on graphene. J. Am. Chem. Soc. 142, 17876–17880 (2020).
Ajaz, A., McLaughlin, E. C., Skraba, S. L., Thamatam, R. & Johnson, R. P. Phenyl shifts in substituted arenes via ipso arenium ions. J. Org. Chem. 77, 9487–9495 (2012).
Skraba-Joiner, S. L., Holt, C. J. & Johnson, R. P. Acid-catalyzed rearrangements in arenes: interconversions in the quaterphenyl series. Beilstein J. Org. Chem. 15, 2655–2663 (2019).
Wan, L., Dastbaravardeh, N., Li, G. & Yu, J.-Q. Cross-coupling of remote meta-C–H bonds directed by a U-shaped template. J. Am. Chem. Soc. 135, 18056–18059 (2013).
Wang, P. et al. Ligand-promoted meta-C–H arylation of anilines, phenols, and heterocycles. J. Am. Chem. Soc. 138, 9269–9276 (2016).
Luo, J., Preciado, S. & Larrosa, I. Overriding ortho–para selectivity via a traceless directing group relay strategy: the meta-selective arylation of phenols. J. Am. Chem. Soc. 136, 4109–4112 (2014).
Liu, L.-Y., Qiao, J. X., Yeung, K.-S., Ewing, W. R. & Yu, J.-Q. meta-C–H arylation of electron-rich arenes: reversing the conventional site selectivity. J. Am. Chem. Soc. 141, 14870–14877 (2019).
Luo, J., Preciado, S. & Larrosa, I. Salicylic acids as readily available starting materials for the synthesis of meta-substituted biaryls. Chem. Commun. 51, 3127–3130 (2015).
Luo, J., Preciado, S., Araromi, S. O. & Larrosa, I. A domino oxidation/arylation/protodecarboxylation reaction of salicylaldehydes: expanded access to meta-arylphenols. Chem. Asian J. 11, 347–350 (2016).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C−H activation for the construction of C−B bonds. Chem. Rev. 110, 890–931 (2009).
Ishikawa, S. & Manabe, K. Synthetic method for multifunctionalized oligoarenes using pinacol esters of hydroxyphenylboronic acids. Chem. Commun. 2006, 2589–2591 (2006).
Yoshida, S., Shimomori, K., Nonaka, T. & Hosoya, T. Facile synthesis of diverse multisubstituted ortho-silylaryl triflates via C–H borylation. Chem. Lett. 44, 1324–1326 (2015).
Sheng, R. et al. Discovery of novel selective GPR120 agonists with potent anti-diabetic activity by hybrid design. Bioorg. Med. Chem. Lett. 28, 2599–2604 (2018).
Izawa, Y., Zheng, C. & Stahl, S. S. Aerobic oxidative Heck/dehydrogenation reactions of cyclohexenones: efficient access to meta-substituted phenols. Angew. Chem. Int. Ed. 52, 3672–3675 (2013).
Vitullo, V. P. & Logue, E. A. Cyclohexadienyl cations. 6. Methyl group isotope effects in the dienone–phenol rearrangement. J. Am. Chem. Soc. 98, 5906–5909 (2002).
Cox, P. A. et al. Base-catalyzed Aryl-B(OH)2 protodeboronation revisited: from concerted proton transfer to liberation of a transient aryl anion. J. Am. Chem. Soc. 139, 13156–13165 (2017).
Picaud, S. et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc. Natl Acad. Sci. USA 110, 19754–19759 (2013).
Sharma, H. A., Mennie, K. M., Kwan, E. E. & Jacobsen, E. N. Enantioselective aryl-iodide-catalyzed Wagner–Meerwein rearrangements. J. Am. Chem. Soc. 142, 16090–16096 (2020).
Bachmann, W. E. & Moser, F. The pinacol–pinacolin rearrangement. The relative migration aptitudes of aryl groups 1. J. Am. Chem. Soc. 54, 1124–1133 (2002).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (2002).
Bassindale, A. R. & Stout, T. The interaction of electrophilic silanes (Me3SiX, X = ClO4, I, CF3SO3, Br, Cl) with nucleophiles. The nature of silylation mixtures in solution. Tetrahedron Lett. 26, 3403–3406 (1985).
Blanch, J. H. Determination of the Hammett substituent constants for the 2-, 3-, and 4-pyridyl and -pyridinium groups. J. Chem. Soc. B 1966, 937–939 (1966).
Leffek, K. T., Llewellyn, J. A. & Robertson, R. E. Some deuterium kinetic isotope effects: IV. β-Deuterium effects in water solvolysis of ethyl, isopropyl and tert-butyl compounds. Can. J. Chem. 38, 2171–2177 (1960).
Cram, D. J. Studies in stereochemistry. I. The stereospecific Wagner–Meerwein rearrangement of the isomers of 3-phenyl-2-butanol. J. Am. Chem. Soc. 71, 3863–3870 (2002).
Phipps, R. J. & Gaunt, M. J. A meta-selective copper-catalyzed C–H bond arylation. Science 323, 1593–1597 (2009).
Yang, G. et al. Pd(II)-catalyzed meta-C–H olefination, arylation, and acetoxylation of indolines using a U-shaped template. J. Am. Chem. Soc. 136, 10807–10813 (2014).
Leitch, J. A. & Frost, C. G. Regioselective transition-metal-catalyzed C–H functionalization of anilines. Synthesis 50, 2693–2706 (2018).
Goodnow, R. A. et al. Discovery of novel and potent leukotriene B4 receptor antagonists. Part 1. J. Med. Chem. 53, 3502–3516 (2010).
Shu, L. et al. Process research on a phenoxybutyric acid LTB4 receptor antagonist. Efficient kilogram-scale synthesis of a 3,5-bisarylphenol core. Org. Process Res. Dev. 17, 114–119 (2012).
Acknowledgements
We acknowledge support from K. Butler, S. Aslam and B. Pointer-Gleadhill from the University of Nottingham Analytical Services. This work was supported by the EPSRC Centre for Doctoral Training in Sustainable Chemistry (grant no. EP/S022236/1, studentship to A.S.), Syngenta (studentship to K.R.), UKRI (grant no. MR/V022067/1, Future Leaders Fellowship to L.T.B.) and the University of Nottingham.
Author information
Authors and Affiliations
Contributions
L.T.B. conceived and directed the project. L.T.B., A.S. and K.R. designed the experiments. A.S. and K.R. carried out the experiments. All the authors analysed the data. L.T.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Tables 1–4, discussion, experimental procedures, characterization data and NMR spectra.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Senior, A., Ruffell, K. & Ball, L.T. meta-Selective C–H arylation of phenols via regiodiversion of electrophilic aromatic substitution. Nat. Chem. 15, 386–394 (2023). https://doi.org/10.1038/s41557-022-01101-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01101-0
- Springer Nature Limited
This article is cited by
-
A para- to meta-isomerization of phenols
Nature Chemistry (2024)