Abstract
Radiotherapy (RT) is a widely used treatment with strong therapeutic effects, but overcoming challenges related to hypoxia-induced tumor resistance and ineffective antitumor immune responses is crucial for optimal outcomes. In this study, we developed a versatile nanosystem using mesoporous silica nanoparticles (MSNs), R837, and a small quantity of manganese peroxide (Mn/ZnO2). The synthesized MSN@R837-Mn/ZnO2 nanoparticles exhibited precise tumor targeting and accumulation, controlled drug release under acidic conditions, and increased sensitivity in magnetic resonance imaging. These attributes collectively augmented the therapeutic efficacy of RT by alleviating hypoxia and immunosuppression. Tumor cells treated with RT combined with these nanoparticles displayed reduced oxidative stress, alleviated hypoxia, and normalized blood vessel formation. Notably, all mice in the RT + PD-1 + MSN@R837-Mn/ZnO2 group achieved complete tumor regression with extended survival. Safety assessments confirmed the absence of MSN@R837-Mn/ZnO2 toxicity, highlighting its potential as a promising approach with dual functionality for the diagnostic imaging and treatment of cancer.
Similar content being viewed by others
Introduction
In cancer management, various approaches are utilized, including surgery, radiotherapy (RT), chemotherapy, and immunotherapy. Among these, RT is a crucial localized treatment modality widely employed within clinical practice1. RT involves the use of radiation, such as external beam radiation or internal radiation implants, to treat cancer by destroying cancer cells or controlling their growth2,3. However, RT is often limited by the emergence of cancer cell resistance to radiation. This resistance, known as RT resistance, refers to a decrease in the effectiveness of cancer treatment. One important challenge in cancer RT is the impact of hypoxia4. Hypoxia affects tissue sensitivity to radiation not only through the well-known ‘oxygen effect’ but also through mechanisms such as reactive oxygen species (ROS) signaling, inflammation, HIF-1-mediated vasculogenesis induction, and alterations in double-strand break repair5. Therefore, it is imperative to devise effective strategies aimed at alleviating tumor hypoxia to bolster the efficacy of RT.
In the past, RT was considered merely a localized treatment method. However, mounting evidence now suggests its potential for activating systemic immunity6. RT can induce the ectopic expression of calreticulin (CRT) on the plasma membrane of tumor cells, acting as an ‘eat me’ signal7. Additionally, the extracellular release of damage-associated molecular patterns (DAMPs), such as HMGB1, promotes the maturation and migration of dendritic cells (DCs) to tumor-draining lymph nodes. This phenomenon is known as immunogenic cell death (ICD) and represents the initial step in establishing an ‘in situ vaccine’. An ideal in situ vaccine would be able to induce immunogenic cell death, expose tumor-reactive antigens, activate antigen-presenting cells (APCs), stimulate a strong and sustained cytotoxic T lymphocyte response, and ultimately elicit a systemic antitumor immune reaction8. As a result, radiation therapy may induce an ‘abscopal effect’, causing tumors at distant sites to also show regression. Nevertheless, radiation therapy rarely impacts distant tumors, suggesting that RT alone is insufficient to trigger a systemic antitumor immune response9. Combining radiation therapy with other immunotherapies may provide a more effective way to exploit its in situ vaccine effect.
Nanomaterials exhibit distinctive properties that can effectively address the limitations of conventional therapies, including the constraints of radiation therapy mentioned earlier. Among nanocarriers, mesoporous silica nanoparticles (MSNs) have garnered substantial interest due to their well-defined pore size and structure, high surface area, biocompatibility, biodegradability, easy surface modification, and stable aqueous dispersion. He et al. developed a novel MSN-based nanovector combining gadolinium oxide (Gd2O3) as a magnetic resonance (MR) imaging contrast agent and doxorubicin (DOX) as an anticancer drug within MSNs10. The nanovector exhibited pH-responsive behavior, releasing DOX in mildly acidic environments, while the incorporation of Gd2O3 substantially enhanced MR imaging contrast with high relaxivity. This approach offers a promising strategy for on-demand drug release and effective MR imaging in theranostics.
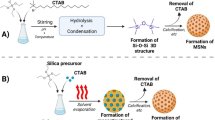
In this work, to mitigate tumor hypoxia and achieve RT-triggered in situ vaccine responses, we developed multifunctional MSN-based nanoparticles through a surfactant-assembled sol-gel process in a Stöber solution (Fig. 1). R837, a Toll-like receptor 7 (TLR7) agonist, was loaded into these nanoparticles. Additionally, a mixture of zinc peroxide and manganese peroxide (Mn/ZnO2) was utilized to seal all the pores in the nanoparticles. The resulting MSN@R837-Mn/ZnO2 nanoparticles, when combined with RT, demonstrated superior tumor-killing efficacy compared to RT alone. This enhanced effect is attributed to the reduction in oxidative stress levels, the alleviation of tumor hypoxia and the normalization of tumor blood vessel formation triggered by zinc ions (Zn2+). Upon RT-induced ICD, tumor cells release tumor-associated antigens. With the aid of R837 as an immune adjuvant, DCs were activated, and macrophages polarized to the M1 phenotype, leading to an increase in effector T cells and a decrease in regulatory T cells (Tregs). Consequently, robust local and systemic antitumor immune responses were induced. With further combination therapy involving anti-PD1 checkpoint blockade, tumor growth were effectively suppressed, and all the mice achieved long-term survival. In addition, the presence of manganese ions (Mn2+) allows for T1-enhanced MR imaging, endowing the MSN@R837-Mn/ZnO2 nanoparticles with dual functionality in the diagnostic imaging and treatment of cancer in vivo.
In vitro, MSNs were synthesized through a surfactant-assembly sol-gel process in a Stöber solution. To encapsulate R837 within the carboxyl-functionalized MSNs, we employed amine-functionalized Mn-doped ZnO2 (Mn/ZnO2) to seal all the pores, creating the final nanoproduct MSN@R837-Mn/ZnO2. In neutral pH environments, the MSN@R837-Mn/ZnO2 nanoparticles maintain their stability. However, in acidic conditions such as in the tumor microenvironment, they undergo degradation, releasing zinc ions (Zn2+), manganese ions (Mn2+) and R837, each of which plays a distinct role within the tumor microenvironment. (1) Zn2+ acts by inhibiting the mitochondrial electron transport chain (ETC), promoting the production of endogenous O2, reducing the local oxidative stress level in the tumor, ameliorating tumor hypoxia, and further increasing the efficacy of RT. (2) Mn2+ enables T1-enhanced magnetic resonance imaging. (3) R837, a Toll-like receptor (TLR) 7 agonist, synergizes with tumor antigens released by RT-induced immunogenic cell death (ICD) and facilitates the activation of DCs and polarization of macrophages toward the M1 phenotype, thereby alleviating the immunosuppressive tumor microenvironment. Consequently, there is an increase in the infiltration of effector T cells and a reduction in regulatory T cells, augmenting the capacity to eliminate tumor cells. In conclusion, our nanoparticles exhibit dual functionality in the diagnostic imaging and treatment of cancer in vivo.
Materials and methods
Materials, cells, and animals
All chemicals were used as received without any additional purification. Ethylene glycol, manganese acetate dihydrate, zinc acetate dihydrate, sodium borohydride, and hydrogen peroxide (H2O2) were purchased from Sinopharm Chemical Reagent Co., Ltd. Cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), 3-aminopropyltriethoxysilane (APTES), succinic anhydride, trimethylamine, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), and COOH-PEG-COOH (MW = 5000) were obtained from Sigma‒Aldrich Chemical Company.
H22 hepatocellular carcinoma cells and 4T1 breast cancer cells were obtained from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology. These cells were cultured with RPMI 1640 medium containing 10% FBS and 1% P/S at 37 °C and 5% CO2. Prior to use, the cells were tested for Mycoplasma contamination, and only Mycoplasma-free cells were used in the experiments.
BALB/c and ICR female mice aged 5–6 weeks were purchased from Shanghai Sippr-BK Laboratory Animal Co., Ltd. (Shanghai, China) and were housed in the specific pathogen-free (SPF) Laboratory Animal Center of the Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School. All animal experimental protocols were approved by the Laboratory Animal Care and Use Committee of the Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School (Approval Number: 2021AE01029).
Synthesis and functionalization of radially-oriented MSNs
Radially-oriented MSNs with nanochannels were synthesized using a surfactant-assembly sol-gel process in a Stöber solution containing CTAB, TEOS, ammonia, and ethanol, as previously described11. To introduce amine groups for functionalization, we utilized APTES as the reagent. The obtained MSNs (300 mg) were then redispersed into a flask with 30 ml of toluene and refluxed for 30 min to ensure optimal nanoparticle dispersion. Subsequently, APTES (300 µl) was added to the refluxing MSN solution, and the mixture was left to react for an additional 10 h. Amine-functionalized MSNs (MSN-NH2) were thoroughly washed with ethanol. Finally, the surface-bound amine moieties were converted into carboxyl groups by dispersing MSN-NH2 in 20 ml of DMSO along with succinic anhydride (100 mg) and triethylamine (100 ml), and the resulting solution was stirred at 50 °C for 48 h.
Synthesis and amine functionalization of Mn-doped ZnO2 (Mn/ZnO2)
Zinc acetate dihydrate (3 mmol) and manganese acetate dehydrate (0.06 mmol) salts were dissolved in ethanol (80 ml), and the solution was refluxed with vigorous stirring. Subsequently, an ethanolic solution (20 ml) of sodium borohydride (7.5 mmol) was introduced, and following the addition of the borohydride solution, 5 ml of 30% H2O2 was added. The addition of H2O2 caused the solution to turn milky. The solution was continuously stirred for an additional 15 min under reflux conditions and then left to reach room temperature. The resulting slurry was diluted by adding 200 ml of boiling water and filtered by vacuum, and the resulting filter cake was washed with 50 ml of hot ethanol to remove byproducts. Mn/ZnO2, as a wet product, was dispersed in anhydrous N,N-dimethylformamide (80 ml). In the next step, 3-aminopropyltriethoxysilane (200 µl) was added to the solution, and the reaction mixture was stirred at 120 °C for 10 min. The resulting amine-functionalized Mn/ZnO2 precipitate was isolated by centrifugation and washed with DMF. Finally, the amine-functionalized Mn/ZnO2 nanoparticles were dispersed in water for further use.
Synthesis of MSN@R837-Mn/ZnO2
For drug loading, carboxyl group-functionalized MSNs (100 mg) were dispersed in 10 ml of a drug solution (2 mg/ml, DMSO) and stirred overnight at room temperature. The resulting R837-loaded MSNs (MSN@R837) were separated by centrifugation to remove any unloaded free drug molecules. To encapsulate the drug within the carboxyl-functionalized MSNs, amine-functionalized Mn/ZnO2 was used, and EDC chemistry was employed for the sealing process. MSN@R837 was redispersed in water, and then 5 mg of EDC and 5 ml of Mn/ZnO2 (10 mg/ml) were added and vortexed for 5 min at room temperature. Upon successful generation of the Mn/ZnO2-sealed MSN@R837 (MSN@R837-Mn/ZnO2) product, COOH-PEG-COOH was further attached to the surface using EDC chemistry to increase the blood circulation time of MSN@R837-Mn/ZnO2.
Characterization
Transmission electron microscopy (TEM) was performed using an FEI Tecnai G2 F20 electron microscope operating at 200 kV. Powder RT-ray diffraction (PXRD) patterns were obtained using a Rigaku D/Max 2550 RT diffractometer with Cu-Kα radiation. Fourier transform infrared (FTIR) spectra were collected using a Nicolet Impact 410 FTIR spectrometer within the range of 400–4000 cm−1. Ultraviolet and visible absorption (UV − VIS) spectra were recorded using a Shimadzu UV-3600 plus spectrophotometer. For the elemental analysis of Zn2+, a Perkin-Elmer Optima 3300DV ICP‒OES was utilized.
In vitro drug release
The pH-responsive behavior of MSN@R837-Mn/ZnO2 was investigated by dialysis of a certain amount of pre-made samples in various pH buffer solutions. The drug release profile was determined by periodic sampling using UV/VIS spectroscopy (detected at 319.6 nm). The drug loading efficiency and drug loading capacity were calculated to be 61% and 12.2%, respectively.
In vitro and in vivo MR imaging
To obtain T1-weighted MR images, samples of MSN@R837-Mn/ZnO2 at different concentrations were placed in a twelve-well plate containing various pH buffer solutions. For the in vivo MRI study, MSN@R837-Mn/ZnO2 nanoparticles (3.33 mg/ml, 200 µl per mouse, n = 3) were intravenously injected into H22 tumor-bearing mice. MR images were acquired at different times.
Animal experiments
H22 hepatocellular carcinoma cells were subcutaneously injected into the left lower abdomen of ICR mice (2 × 106 cells per mouse, Day 0). Approximately 5 days later, when the tumor size reached almost 100 mm3, the mice were randomly divided into eleven groups: the NS group (200 μl/mouse), MSN group, MSN-Mn/ZnO2 group, MSN@R837-Mn/ZnO2 group (200 μg/200 μl/mouse), RT group (5 Gy/mouse), RT + MSN group, RT + MSN-Mn/ZnO2 group, RT + MSN@R837-Mn/ZnO2 group, PD-1 group (100 μg/mouse), RT + PD-1 group, and RT + PD-1 + MSN@R837-Mn/ZnO2 group. The nanoparticle treatment was administered on Day 5 through intravascular injection, followed by RT irradiation 4 h after injection and anti-PD1 antibody treatment was administered on Days 7, 9 and 11. The tumor sizes and body weights were measured once every 2 or 3 days, and the mice were euthanized when the lengths of tumors reached 1.5 cm. On Day 5, 4 h after nanoparticle treatment or RT, the tumors were collected for immunofluorescence staining. On Day 15, the following serum samples were collected to assay the following biochemical indicators: aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine (Cr). Hearts, livers, lungs and kidneys were excised for hematoxylin–eosin staining. Spleens, lymph nodes and tumors were also collected for flow cytometry on Day 15.
Similarly, 4T1 breast cancer cells were subcutaneously injected into the left lower abdomen of BALB/c mice (2 × 106 cells per mouse, Day 0). After approximately 5 days, when the tumor size reached almost 100 mm3, the mice were randomly divided into five groups: the NS group (200 μl per mouse), RT group (5 Gy per mouse), RT + MSN group, RT + MSN-Mn/ZnO2 group, and RT + MSN@R837-Mn/ZnO2 group (200 μg dissolved in 200 μl of NS per mouse). The nanoparticle treatment was administered on Day 5 through intravascular injection, followed by RT irradiation 4 h after injection. The tumor sizes and body weights were measured once every 2 or 3 days, and the mice were euthanized when the lengths of the tumors reached 1.5 cm.
Therapeutic efficacy of MSN@R837-Mn/ZnO2 in patient-derived organoids
Two pathological types of human gastric cancer tissues, namely, mucous adenocarcinoma and low-adhesion carcinoma, were obtained from the Pathology Department of the Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School for the culture of patient-derived organoids (PDOs). PDOs were established following previously described culture methods12,13,14. Organoids were harvested from the Matrigel using 1x TrypLE (Gibco) and dissociated into small clusters for drug response analysis. Subsequently, the organoids were resuspended in 2% Matrigel/organoid culture medium (200–1000 clusters/ml) and dispensed into 384-well plates in triplicate. The dose of MSN@R837-Mn/ZnO2 used in the experiment was 100 µg/ml. To analyze the drug response, a CellTiter-Glo 3D Cell Viability Assay (Promega) was used according to the manufacturer’s instructions after 14 days of different treatments, and the results were normalized to those of vehicle controls. This research involving human participants was approved by the Research Ethics Committee of the Comprehensive Cancer Center of Drum Tower Hospital (Ethics approval number/ID: 2021–324). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Prior to participation in the study, written informed consent was obtained from all patients involved.
Immunofluorescence staining
Tumor sections were prepared and incubated with specific fluorescent probes and antibodies to visualize various cellular markers. Zinquin ethyl ester (J&K Scientific, China) was used to stain zinc ions, while DCFH-DA (Sigma‒Aldrich, USA) was used to detect ROS. A Hypoxyprobe Green Kit (HPI Inc., USA) was utilized to label the hypoxic areas. Additionally, an anti-CD31 rat monoclonal antibody (1:200) (Abcam, UK) and an anti-calreticulin rabbit monoclonal antibody (1:200) (Abcam, UK) were applied to detect CD31-expressing blood vessels and ICDs, respectively. The tumor sections were incubated with these probes and antibodies overnight at 4 °C. After thorough washing with PBS three times, the sections were further stained with secondary antibodies to detect the primary antibody-bound targets. Specifically, a goat anti-rat IgG H&L (Cy5, 1:200) (Abcam, UK) and a goat anti-rabbit IgG H&L (Cy3, 1:200) (Abcam, UK) were used as secondary antibodies. DAPI (Sangon Biotech, China) was used as a nuclear counterstain. To preserve the fluorescence signals, the sections were sealed with 50% glycerol. Subsequently, fluorescence images were captured using a confocal microscope (Leica, Germany) to visualize and analyze the specific markers and cellular components within the tumor sections.
Flow cytometry
Single-cell suspensions were prepared as follows: the lymph nodes and spleens were mechanically dissociated, filtered, and suspended in NS (0.5–1 × 106 cells/ml). Tumors were cut into small pieces, incubated with collagenase type IV (1 mg/ml, Sigma, USA) at 37 °C for 3–4 h, filtered, and suspended in NS (0.5–1 × 106 cells/ml). As most tested antigens are expressed on cell membranes, the samples were stained with specific antibodies for 20 min at 4 °C in the dark and then washed before analysis. For Foxp3, which is expressed in the nucleus, the True-Nuclear Transcription Factor Buffer Set (Biolegend, USA) was used. The following monoclonal antibodies obtained from Biolegend were used for flow cytometry: CD11c-FITC (5 μg/ml), CD80-APC (2 μg/ml), CD86-PE (2 μg/ml), CD11b-FITC (5 μg/ml), F4/80-PE-Cy7 (2 μg/ml), CD206-APC (2 μg/ml), CD3-FITC (5 μg/ml), CD4-PE-Cy7 (2 μg/ml), CD8-PE-Cy5 (2 μg/ml), CD44-PE (2 μg/ml), CD62L-APC (2 μg/ml), CD25-APC (2 μg/ml), and Foxp3-PE (2 μg/ml). To measure the levels of IL-6 and IL-10, a BD™ Cytometric Bead Array (CBA), Mouse Interleukin (IL)-6 Flex Set, and Mouse IL-10 Flex Set were used for detection and analysis. By employing these techniques and reagents, we were able to analyze immune cell populations, surface marker expression, and cytokine levels within single-cell suspensions obtained from lymph nodes, spleens, and tumors.
Cytotoxicity assay of mouse splenocytes
H22 hepatocellular carcinoma cells were labeled with CFSE for 10 min at 37 °C in the dark. Subsequently, splenocytes from mice in the four groups (RT group, RT + MSN group, RT + MSN-Mn/ZnO2 group, and RT + MSN@R837-Mn/ZnO2 group) were cocultured with CFSE-labeled H22 hepatocellular carcinoma cells at different effector-to-target ratios (E:T) of 5:1, 10:1, and 20:1. The cocultures were maintained at 37 °C and 5% CO2. After 6 h, the cells were stained with propidium iodide (PI) for 20 min at 4 °C in the dark and then washed prior to analysis.
Statistical analyses
Unless otherwise specified, all experiments were performed with biological replicates. Paired two-tailed Student’s t tests were used for comparisons between two groups. For comparisons among multiple groups, one-way analysis of variance (ANOVA) or two-way ANOVA was applied. Survival benefit was assessed using the log-rank test. Statistical significance is denoted as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
Results
Generation and characterization of MSN@R837-Mn/ZnO2
A classical surfactant-assembly sol-gel method (Stöber solution containing CTAB, TEOS, ammonia, and ethanol) was utilized to prepare radially-oriented MSNs11. To encapsulate R837 within the carboxyl-functionalized MSNs, we employed amine-functionalized 2% Mn-doped ZnO2 (Mn/ZnO2) to seal all the pores, resulting in the formation of the final nanoproduct, MSN@R837-Mn/ZnO2. The TEM images revealed the well-defined spherical shape of the synthesized MSN@R837-Mn/ZnO2, with the R837-loaded portion located at the spherical center, characterized by its dark color, and the Mn/ZnO2 was distributed uniformly at the edges (Fig. 2a). The nanoparticles displayed homogeneous sizes of approximately 300 nm. PXRD patterns further confirmed the successful synthesis of the MSN@R837-Mn/ZnO2 nanoparticles (Fig. 2b). Following optimization, we calculated the final loading efficiency and loading capacity of R837 to be 61% and 12.2%, respectively. As control samples, we prepared MSN, Mn/ZnO2, and MSN-Mn/ZnO2 nanoparticles using a similar method.
a Transmission electron microscopy (TEM) images of mesoporous silica nanoparticles (MSNs, scale bars: 1 µm and 50 nm), 2% Mn-doped ZnO2 (Mn/ZnO2, scale bars: 50 nm and 5 nm) and MSN@R837-Mn/ZnO2 (scale bars: 50 nm and 5 nm). b Powder RT diffraction (PXRD) patterns of MSNs, Mn/ZnO2 and MSN@R837-Mn/ZnO2. c Curves depicting the release of R837 from MSN@R837-Mn/ZnO2 in different pH buffer solutions. d Histograms illustrating the release of zinc ions (Zn2+) from MSN@R837-Mn/ZnO2 in different pH buffer solutions. e Histograms depicting the release of hydrogen peroxide (H2O2) from MSN@R837-Mn/ZnO2 in different pH buffer solutions.
Enhanced T1-weighted MR imaging characteristics of MSN@R837-Mn/ZnO2
In neutral environments (pH = 7.4), the MSN@R837-Mn/ZnO2 nanoparticles demonstrated excellent stability (Fig. 2c–e). However, under acidic conditions (pH = 5.5), they undergo controlled degradation, releasing Zn2+, H2O2, and small amounts of Mn2+ and R837. We carefully investigated the pH-responsive release behavior of R837, Zn2+ and H2O2 using UV/VIS spectroscopy and revealed that approximately 50% of the Zn2+ and H2O2 were released from MSN@R837-Mn/ZnO2 within 1 h, while approximately 50% of the R837 was released within 40 h. This release of components caused the MSN@R837-Mn/ZnO2 solution to change color under different pH conditions, and the released components were observed as fragments in the TEM image (Fig. 3a).
a Images of MSN@R837-Mn/ZnO2 in different pH buffer solutions and a TEM image in HAc-NAc buffer (pH = 5.5). b In vitro T1-weighted MR images of MSN@R837-Mn/ZnO2 at various concentrations in different pH buffer solutions. c In vivo T1-weighted MR images of tumor-bearing mice at different time points after intravenous injection of MSN@R837-Mn/ZnO2 (3.33 mg/ml, 200 µl per mouse, n = 3).
Compared to that using the nanoparticles in water and solutions with the same concentration of neutral solutions, T1-weighted MR imaging using the nanoparticles in acidic solutions of varying concentrations was significantly enhanced, and the brightness increased as the concentration increased (Fig. 3b). This enhancement is attributed to the release of Mn2+ under acidic conditions. Furthermore, T1-weighted MR imaging confirmed the accumulation of MSN@R837-Mn/ZnO2 in the tumor area (Fig. 3c). In tumor-bearing mice, a distinct contrast in the tumor area was observed at 2 h and 6 h posttreatment, validating the effective delivery of intravenously injected MSN@R837-Mn/ZnO2 into the tumor. Thus, these nanoparticles serve as promising contrast agents for imaging-guided tumor therapy.
Antitumor efficacy of MSN@R837-Mn/ZnO2
The antitumor effect of MSN-based nanoparticles was validated both in vitro and in vivo. In vitro, as determined by the CCK-8 test, MSNs or R837 alone exerted no cytotoxic effects on H22 hepatocellular carcinoma cells even at concentrations as high as 0.2 mg/ml, whereas MSN-Mn/ZnO2 and MSN@R837-Mn/ZnO2 demonstrated a dose-dependent ability to kill tumor cells (Fig. 4a). For in vivo evaluation, we established a hepatocellular carcinoma-bearing mouse model and randomly divided the mice into four groups: the NS group, MSN group, MSN-Mn/ZnO2 group and MSN@R837-Mn/ZnO2 group. The treatment scheme is illustrated in Fig. 4b. Notably, as shown in Fig. 4c, tumor growth progressed rapidly in the NS group and MSN group. In comparison, tumor growth in the MSN-Mn/ZnO2 group was slightly slower than that in the first two groups, while significant tumor growth inhibition was observed in the MSN@R837-Mn/ZnO2 group. Furthermore, images of tumors harvested on the 15th day post tumor inoculation revealed the smallest tumors in the MSN@R837-Mn/ZnO2 group at this time point (Fig. 4e). Consistent with these findings, the median survival time of mice in the MSN@R837-Mn/ZnO2 group was 2.27 times that of those in the NS and MSN groups (p < 0.0001) and 1.55 times that of those in the MSN-Mn/ZnO2 group (p = 0.0004, Fig. 4d).
a Relative viability of H22 hepatocellular carcinoma cells after incubation with various concentrations of MSNs, R837, MSN-Mn/ZnO2 and MSN@R837-Mn/ZnO2, as determined by a CCK8 assay. The data are presented as the mean ± SEM. P values were determined by two-way ANOVA with Tukey’s HSD multiple comparison post hoc test. ***P < 0.0001 (MSN-Mn/ZnO2 group, NS vs. 0.05 mg/ml, NS vs. 0.1 mg/ml, NS vs. 0.2 mg/ml), *P = 0.0187 (MSN-Mn/ZnO2 group, 0.01 mg/ml vs. 0.05 mg/ml), ***P < 0.0001 (MSN@R837-Mn/ZnO2 group, NS vs. 0.05 mg/ml, NS vs. 0.1 mg/ml, NS vs. 0.2 mg/ml), *P = 0.0399 (MSN@R837-Mn/ZnO2 group, 0.01 mg/ml vs. 0.05 mg/ml). b Treatment schema of MSN@R837-Mn/ZnO2. ICR mice were implanted with H22 hepatocellular carcinoma cells (2 × 106) on the left lower skin of the abdomen on Day 0. Approximately 5 days later, when the tumor size reached almost 100 mm3, the mice received intravascular injections of NS, MSNs, MSN-Mn/ZnO2 or MSN@R837-Mn/ZnO2. c Growth curves representing the average tumor volumes of each group (n = 6). The data are presented as the mean ± SEM. P values were determined by two-way ANOVA with Tukey’s HSD multiple comparison post hoc test. ***P < 0.0001 (NS vs. MSN-Mn/ZnO2), ***P = 0.0004 (MSN-Mn/ZnO2 vs. MSN@R837-Mn/ZnO2). d Survival curves (n = 6). P values were determined by the log-rank (Mantel‒Cox) test. **P = 0.0022 (MSN vs. MSN-Mn/ZnO2), ***P = 0.0008 (MSN-Mn/ZnO2 vs. MSN@R837-Mn/ZnO2). e Photos of tumors harvested from mice in all groups on the 15th day after tumor inoculation (n = 6). f Bright-field microscopy images of patient-derived organoids (PDOs) from two pathological types of gastric cancer treated with NSs, MSNs, MSN-Mn/ZnO2 or MSN@R837-Mn/ZnO2. Mu represents mucous adenocarcinoma, and La represents low-adhesion carcinoma. g Inhibition rate of MSN-Mn/ZnO2 and MSN@R837-Mn/ZnO2 on PDOs in mice. The data are presented as the mean ± SEM. P values were calculated by one-way ANOVA with Tukey’s multiple comparisons test. ***P < 0.0001 (NS vs. MSN-Mn/ZnO2, NS vs. MSN@R837-Mn/ZnO2, MSN-Mn/ZnO2 vs. MSN@R837-Mn/ZnO2). h Inhibition rate of MSN-Mn/ZnO2 and MSN@R837-Mn/ZnO2 on PDOs in La. The data are presented as the mean ± SEM. P values were calculated by one-way ANOVA with Tukey’s multiple comparisons test. ***P < 0.0001 (NS vs. MSN@R837-Mn/ZnO2, MSN-Mn/ZnO2 vs. MSN@R837-Mn/ZnO2).
To assess the potential clinical applications of these MSN-based nanoparticles, we conducted experiments using PDOs derived from two pathological types of gastric cancer: mucous adenocarcinoma and low-adhesion carcinoma. In PDOs of mucinous carcinoma, the MSN@R837-Mn/ZnO2 group exhibited a remarkable inhibition rate of 88.22%, whereas the inhibition rate was only 38.23% in the MSN-Mn/ZnO2 group (Fig. 4f, g). Conversely, in PDOs of low-adhesion carcinoma, the MSN@R837-Mn/ZnO2 group demonstrated an inhibition rate of 64.67%, while MSN-Mn/ZnO2 had no discernible cytotoxic effect (Fig. 4f, h). These compelling results indicate that MSN@R837-Mn/ZnO2 also exerts antitumor effects on human tumors, supporting its potential for further investigation in clinical applications.
Hypoxia alleviation characteristics of MSN@R837-Mn/ZnO2
Given the ability of Zn2+ to inhibit the mitochondrial electron transport chain (ETC) and facilitate the generation of endogenous H2O2 and O2, we investigated the potential of MSN@R837-Mn/ZnO2 to alleviate tumor hypoxia in vivo using an immunofluorescence staining assay (Fig. 5). ICR mice bearing H22 tumors were sacrificed, and their tumors were collected 4 h after the injection of different nanoparticles for immunofluorescence staining. Notably, compared to those in the tumor tissue of mice injected with NS or MSN, a greater number of cells in the tumor tissue of mice injected with MSN-Mn/ZnO2 or MSN@R837-Mn/ZnO2 exhibited uptake of Zn2+ (Fig. 5a, b). Moreover, pronounced decreases in both ROS signals (detected by the ROS probe DCFH-DA) and hypoxia signals (detected by pimonidazole) were observed in the tumors of mice treated with MSN-Mn/ZnO2 or MSN@R837-Mn/ZnO2 (Fig. 5c–f). As a result, the number of thicker and disorderly blood vessels in the tumors significantly decreased in these two groups (Fig. 5g, h).
ICR mice were implanted with H22 hepatocellular carcinoma cells (2 × 106) on the left lower skin of the abdomen on Day 0. Approximately 5 days later, when the tumor size reached almost 100 mm3, the mice received intravascular injections of NS, MSNs, MSN-Mn/ZnO2 or MSN@R837-Mn/ZnO2 (n = 6). Tumors harvested from mice in all groups 4 h postinjection were sectioned and subjected to immunofluorescence staining. a Cells that took up zinc ions (Zn2+) are stained red. Scale bars, 166.4 μm. b Proportion of Zn2+ cells relative to the total number of cells in the tumor (n = 5 section images). The data are presented as the mean ± SEM. P values were calculated by one-way ANOVA with Tukey’s multiple comparisons test. *P = 0.0309 (NS vs. MSN-Mn/ZnO2), *P = 0.0278 (MSN vs. MSN-Mn/ZnO2), **P = 0.0085 (NS vs. MSN@R837-Mn/ZnO2), **P = 0.0076 (MSN vs. MSN@R837-Mn/ZnO2). c Reactive oxygen species (ROS) in cells were detected with DCFH-DA (red). Scale bars, 166.4 μm. d Proportion of ROS+ cells relative to the total number of cells in the tumor (n = 5 section images). The data are presented as the mean ± SEM. P values were calculated by one-way ANOVA with Tukey’s multiple comparisons test. **P = 0.0018 (MSN vs. MSN@R837-Mn/ZnO2), **P = 0.0036 (MSN vs. MSN-Mn/ZnO2), ***P < 0.0001 (NS vs. MSN-Mn/ZnO2, NS vs. MSN@R837-Mn/ZnO2). e Hypoxic areas were stained with an anti-pimonidazole antibody (green). Scale bars, 166.4 μm. f Proportion of hypoxic areas after treatment (n = 5 section images). The data are presented as the mean ± SEM. P values were calculated by one-way ANOVA with Tukey’s multiple comparisons test. ***P = 0.0008 (NS vs. MSN-Mn/ZnO2), **P = 0.0081 (MSN vs. MSN-Mn/ZnO2), ***P = 0.0001 (NS vs. MSN@R837-Mn/ZnO2), ***P = 0.0010 (MSN vs. MSN@R837-Mn/ZnO2). g Blood vessels were stained with an anti-CD31 antibody (red). Scale bars, 166.4 μm. h Proportion of blood vessel area after treatment (n = 5 section images). The data are presented as the mean ± SEM. P values were calculated by one-way ANOVA with Tukey’s multiple comparisons test. ***P = 0.0003 (NS vs. MSN), *P = 0.0156 (MSN vs. MSN@R837-Mn/ZnO2), ***P < 0.0001 (NS vs. MSN-Mn/ZnO2, NS vs. MSN@R837-Mn/ZnO2).
Antitumor efficacy of MSN@R837-Mn/ZnO2 combined with RT
The preceding experiments demonstrated that MSN@R837-Mn/ZnO2 can ameliorate tumor hypoxia, suggesting its potential to enhance RT efficacy. To investigate the combined efficacy of MSN@R837-Mn/ZnO2 with an anti-PD1 antibody, we established two mouse models: a hepatocellular carcinoma model and a breast cancer model. In the hepatocellular carcinoma mouse model, tumor-bearing mice were randomly divided into four treatment groups: the RT group, RT + MSN group, RT + MSN-Mn/ZnO2 group, and RT + MSN@R837-Mn/ZnO2 group, as illustrated in Fig. 6a. Notably, tumor growth in the RT + MSN@R837-Mn/ZnO2 group was significantly inhibited, with one out of six mice achieving complete tumor regression (Fig. 6b). Moreover, the median survival time of the mice in the RT + MSN@R837-Mn/ZnO2 group exceeded 45 days, while those in the other three groups survived for only 22, 23.5, and 28 days, respectively (Fig. 6c). Similar results were obtained in the breast cancer model (Fig. 6d, e).
a Treatment schema for both hepatocellular carcinoma and breast cancer mouse models. ICR or BALB/c mice were implanted with H22 hepatocellular carcinoma cells (2 × 106) or 4T1 breast cancer cells (2 × 106), respectively, on the left lower skin of the abdomen on Day 0. Approximately 5 days later, when the tumor size reached almost 100 mm3, the mice were randomly assigned to receive intravascular injection of NS, MSN, MSN-Mn/ZnO2 or MSN@R837-Mn/ZnO2, followed by RT radiation at a dose of 5 Gy, which was administered 4 h after injection. b Growth curves representing the average tumor volumes of each group in the hepatocellular carcinoma mouse model (n = 6). The data are presented as the mean ± SEM. P values were determined by two-way ANOVA with Tukey’s HSD multiple comparison post hoc test. ***P = 0.0003 (RT vs. RT + MSN), ***P < 0.0001 (RT + MSN vs. RT + MSN-Mn/ZnO2, RT + MSN-Mn/ZnO2 vs. RT + MSN@R837-Mn/ZnO2). c Survival curves of the hepatocellular carcinoma mouse model (n = 6). P values were determined by the log-rank (Mantel‒Cox) test. *P = 0.029 (RT vs. RT + MSN), **P = 0.0095 (RT + MSN vs. RT + MSN-Mn/ZnO2), ***P = 0.0008 (RT + MSN-Mn/ZnO2 vs. RT + MSN@R837-Mn/ZnO2). d Growth curves representing the average tumor volumes of each group in the breast cancer mouse model (n = 6). The data are presented as the mean ± SEM. P values were determined by two-way ANOVA with Tukey’s HSD multiple comparison post hoc test. ***P = 0.0001 (NS vs. RT), **P = 0.0014 (RT + MSN-Mn/ZnO2 vs. RT + MSN@R837-Mn/ZnO2), ***P < 0.0001 (RT + MSN vs. RT + MSN@R837-Mn/ZnO2). e Survival curves in the breast cancer mouse model (n = 6). P values were determined by the log-rank (Mantel‒Cox) test. ***P = 0.0007 (RT + MSN vs. RT + MSN@R837-Mn/ZnO2). Representative images and quantitative data of cells that took up zinc ions (f), reactive oxygen species (ROS) in cells (g), hypoxic areas (h), and blood vessels (i) are shown. Scale bars, 166.4 μm. The data are presented as the mean ± SEM. P values were calculated by unpaired Student’s t test. *P = 0.0225 (f), **P = 0.0029 (g), ***P < 0.0001 (h), ***P = 0.0002 (i). (n = 5 section images).
Compared to tumors in the RT group, tumors in the combination treatment RT and MSN@R837-Mn/ZnO2 group exhibited several key changes, as shown in Fig. 6f–i. Specifically, the tumor cells displayed increased absorption of Zn2+ (Fig. 6f), resulting in lower levels of oxidative stress (Fig. 6g). This process facilitated the generation of endogenous O2, which in turn significantly alleviated tumor hypoxia (Fig. 6h) and led to the development of thinner and normally organized blood vessels (Fig. 6i). Collectively, these factors contribute to the enhanced RT efficacy achieved by MSN@R837-Mn/ZnO2.
Immune responses induced by MSN@R837-Mn/ZnO2 combined with RT
The induction of ICD in cancer cells is characterized by the elevated expression of CRT on the surface of dying cancer cells. To assess this phenomenon, tumor sections from each treatment group were subjected to confocal imaging after RT irradiation at a dose of 5 Gy. Notably, CRT was clearly expressed on the cell surface in both the RT and RT + MSN@R837-Mn/ZnO2 groups (Fig. 7a). This observation indicates that RT effectively induces ICD in cancer cells, leading to the generation of tumor-associated antigens and thus fostering antitumor immune responses.
a The immunogenic cell death marker calreticulin (CRT) was detected by staining with an anti-CRT antibody (red), and the proportion of CRT+ cells relative to the total number of cells in the tumor was calculated (n = 5 section images). Scale bars, 83.2 μm. **P = 0.0015, ***P = 0.0005. b Percentage of mature DCs (mDCs, CD11c+CD80+CD86+) derived from bone marrow-derived cells (BMDCs) in vitro. ***P < 0.0001. c Percentage and representative flow cytometry images of mDCs in the tumor. *P = 0.0125, **P = 0.0033 (RT vs. RT + MSN@R837-Mn/ZnO2), **P = 0.0026 (RT + MSN vs. RT + MSN@R837-Mn/ZnO2). d Percentage of mDCs in the spleen. **P = 0.0042, ***P = 0.0007 (RT vs. RT + MSN@R837-Mn/ZnO2), ***P = 0.0009 (RT + MSN vs. RT + MSN@R837-Mn/ZnO2). e Percentage and representative flow cytometry images of M1-like macrophages (M1, CD11b+F4/80+CD86+) in tumors. *P = 0.0219, **P = 0.0047. f Percentage of M2-like macrophages (M2, CD11b+F4/80+CD206+) in the tumor. *P = 0.0146, **P = 0.0089. g Percentage and representative flow cytometry images of effector memory T cells (TEM, CD3+CD8+CD44+CD62L–) in the spleen. *P = 0.0272, **P = 0.0075 (RT vs. RT + MSN@R837-Mn/ZnO2), **P = 0.0037 (d, RT + MSN vs. RT + MSN@R837-Mn/ZnO2). h Splenocytes from mice in each group were isolated and incubated with CFSE-labeled H22 hepatocellular carcinoma cells at effector-to-target ratios (E:T) of 5:1, 10:1, and 20:1. PI was added after 6 h of incubation, and the percentage of dead cells (CFSE+PI+) was analyzed using flow cytometry (n = 3). P values were determined by two-way ANOVA with Tukey’s HSD multiple comparison post hoc test. **P = 0.0026, ***P = 0.0002 (RT vs. RT + MSN-Mn/ZnO2), ***P < 0.001 (RT + MSN-Mn/ZnO2 vs. RT + MSN@R837-Mn/ZnO2). i Percentage and representative flow cytometry images of regulatory T cells (Tregs, CD3+CD4+CD25+FOXP3+) in tumors. ***P = 0.0004 (RT + MSN-Mn/ZnO2 vs. RT + MSN@R837-Mn/ZnO2), ***P < 0.001 (RT vs. RT + MSN-Mn/ZnO2, RT vs. RT + MSN@R837-Mn/ZnO2). j Levels of IL-6 and IL-10 in tumors. *P = 0.0208 (IL-6), *P = 0.0158 (IL-10), **P = 0.0064 (RT + MSN-Mn/ZnO2 vs. RT + MSN@R837-Mn/ZnO2), **P = 0.0035 (RT vs. RT + MSN@R837-Mn/ZnO2). For all quantitative data except (h), the data are presented as the mean ± SEM (n = 3). P values were calculated by one-way ANOVA with Tukey’s multiple comparisons test.
DCs, the most crucial type of antigen-presenting cell, play a pivotal role in activating and regulating innate and adaptive immunity. In this study, bone marrow-derived cells (BMDCs) were obtained from ICR mice and incubated in vitro with NS, MSN-Mn/ZnO2, R837, or MSN@R837-Mn/ZnO2 nanoparticles for 48 h. The percentage of mature DCs (mDCs), as determined by flow cytometry, was found to be similar when the cells were incubated with MSN@R837-Mn/ZnO2 or free R837 at the same dose. In contrast, MSN-Mn/ZnO2 without R837 exhibited no significant stimulatory effect on BMDCs (Fig. 7b, Fig. S1a). Consistent with the in vitro findings, the in vivo results revealed that the percentage of mDCs in the tumors of the RT + MSN@R837-Mn/ZnO2 group was 2.8 times greater than that in the tumors of the RT group (Fig. 7c), while in the spleen, it was 2 times greater (Fig. 7d, Fig. S1b).
Hypoxia within the tumor microenvironment has been associated with the recruitment of a large number of macrophages and their polarization toward the M2 phenotype, which promotes tumor growth. Conversely, the alleviation of hypoxia in the TME following MSN@R837-Mn/ZnO2 treatment may have regulatory effects on tumor-associated macrophages (TAMs). Notably, after the combination treatment of RT and MSN@R837-Mn/ZnO2, a significant increase in the M1 phenotype and a decrease in the M2 phenotype of TAMs were observed (Fig. 7e, f, Fig. S1c–g). Moreover, the downregulation of interleukin 10 (IL-10) in the tumors of the RT + MSN@R837-Mn/ZnO2 group further contributed to the polarization of TAMs from the M2 to M1 phenotype (Fig. 7j).
In response to the antigen-presenting action of mDCs and M1-like macrophages, a substantial number of effector T cells were activated in both the spleen and lymph nodes of the RT + MSN@R837-Mn/ZnO2 group, with a 1.4-fold increase in the spleen and a 1.7-fold increase in the lymph nodes compared to those in the RT group (Fig. 7g, Fig. S2a). To further assess the functionality of these effector T cells, we examined the tumor-killing ability of spleen lymphocytes. Figure 7h and Fig. S2b demonstrate that, regardless of the effector target ratio (number of spleen lymphocytes: number of H22 tumor cells), the highest proportion of dead tumor cells was observed after incubation with spleen lymphocytes from the RT + MSN@R837-Mn/ZnO2 group. Within the tumor microenvironment, the RT + MSN@R837-Mn/ZnO2 group exhibited significant downregulation of Tregs and IL-6 (Fig. 7i, j). This suggests that the remarkable alleviation of tumor hypoxia by our nanoparticles not only overcomes hypoxia-associated radiation resistance but also reverses the immunosuppressive nature of the tumor microenvironment, synergistically enhancing the effectiveness of cancer treatment.
Enhanced antitumor efficacy of MSN@R837-Mn/ZnO2 combined with RT and PD-1 blockade
To further augment the therapeutic potential of MSN@R837-Mn/ZnO2 combined with RT, we focused on an anti-PD1 antibody, a widely used immunotherapy drug in clinical practice. In this study, we utilized the same hepatocellular carcinoma mouse model as previously described and administered anti-PD1 antibody treatment starting 2 days after nanoparticle injection and RT irradiation, with subsequent doses given once every other day, totaling three doses (Fig. 8a). Among the groups tested, with the exception of the PD-1 group, all the other groups demonstrated significant tumor suppression effects (Fig. 8b). Notably, all mice in the RT + PD-1 + MSN@R837-Mn/ZnO2 group achieved complete tumor regression and exhibited prolonged survival (Fig. 8c).
a Treatment schema. ICR mice were implanted with H22 hepatocellular carcinoma cells (2 × 106) on the left lower skin of the abdomen on Day 0. Approximately 5 days later, when the tumor size reached almost 100 mm3, the mice were randomly assigned to receive intravascular injection of NS, MSN, MSN-Mn/ZnO2 or MSN@R837-Mn/ZnO2, followed by RT irradiation 4 h after injection and anti-PD1 antibody treatments on Days 7, 9 and 11. b Growth curves representing the average tumor volumes of each group (n = 4). The data are presented as the mean ± SEM. P values were determined by two-way ANOVA with Tukey’s HSD multiple comparison post hoc test. **P = 0.0022, ***P < 0.0001 (PD-1 vs. RT + MSN@R837-Mn/ZnO2, PD-1 vs. RT + PD-1 + MSN@R837-Mn/ZnO2). c Survival curves (n = 4). P values were determined by the log-rank (Mantel‒Cox) test. **P = 0.0031 (PD-1 vs. RT + MSN@R837-Mn/ZnO2), **P = 0.0067 (PD-1 vs. RT + PD-1 + MSN@R837-Mn/ZnO2). d Hematology indices of mice treated with NSs, MSNs, MSN-Mn/ZnO2 or MSN@R837-Mn/ZnO2 (n = 4). The data are presented as the mean ± SEM. P values were determined by two-way ANOVA with Tukey’s HSD multiple comparison post hoc test. e Hematoxylin–eosin staining of the main organs, including the heart, kidney, liver and lung. Scale bars, 50 μm. f, g Body weights of mice treated with intravascular injection of nanoparticles combined with or without RT (n = 6).
Biosafety assessment
To comprehensively evaluate the biosafety of MSN@R837-Mn/ZnO2, we conducted serum biochemistry assays on ICR mice from four different groups: the NS, MSN, MSN-Mn/ZnO2, and MSN@R837-Mn/ZnO2 groups. The AST, ALT, BUN, and Cr levels were comparable among the four groups and remained within the normal range (Fig. 8d). These findings suggest that intravascular injection of MSN@R837-Mn/ZnO2 did not significantly affect liver or kidney function in the mice. Furthermore, histological examination of major organs, as shown in Fig. 8e, did not indicate any abnormalities or adverse effects. During the treatment of H22 mouse hepatocellular carcinoma, the mice in all groups, except for the NS group, did not display any abnormal fluctuations in body weight (Fig. 8f, g). This observation further supports the suitable biological safety profile of the MSN@R837-Mn/ZnO2 nanoparticles. Taken together, our biosafety assessments indicate that the MSN@R837-Mn/ZnO2 nanoparticles demonstrate favorable biocompatibility and are well tolerated, making them promising candidates for future clinical applications.
Discussion
To increase the effectiveness of RT, we developed a multifunctional MSN@R837-Mn/ZnO2 nanoparticle with three key features: (1) passive accumulation in tumor tissues via the enhanced permeability and retention (EPR) effect, allowing controlled drug release in acidic environments to enhance the signal intensity in T1-weighted MR images; (2) alleviation of tumor hypoxia and promotion of tumor vascular normalization; and (3) combination with RT to induce the generation of an in situ vaccine within the tumor, thereby activating a systemic antitumor immune response.
The physicochemical properties of nanoparticles, including surface area, shape, and size, significantly impact their drug delivery efficiency15. MSNs are known for their high specific surface area (>1000 m2/g), enabling efficient loading of therapeutic molecules. Compared to the FDA-approved liposomal formulation Doxil®, MSNs can load nearly 1000 times more DOX16. Studies have shown that rod-shaped MSNs exhibit the greatest cellular uptake, followed by spherical MSNs17. For optimal circulation half-lives, MSNs are preferred to be in the size range of 50–300 nm, as sizes within this range prevent rapid renal clearance and potential embolisms caused by capillary and alveolar aggregation18. In the physiological environment, MSNs form a protein corona due to interactions with serum proteins, which alters in vitro parameters such as size and surface charge, affecting cellular uptake and leading to clearance by the mononuclear phagocyte system19. Coating MSNs with PEG can prevent this phenomenon, extending the circulation time of the nanoparticles in the bloodstream20. Utilizing the aforementioned theory, we engineered spherical nanoparticles with a size of approximately 300 nm (Fig. 2a). R837 was encapsulated within the spherical core through the incorporation of a small quantity of manganese peroxide into zinc peroxide. The inclusion of peroxides rendered the nanoparticles pH-responsive, facilitating swift degradation into zinc hydroxide, manganese hydroxide, and hydrogen peroxide within weakly acidic environments (Fig. 2d, e). As a result, R837 was gradually released from the nanoparticle core. This controlled release mechanism occurs at a comparatively slow rate (Fig. 2c). In the mildly acidic tumor microenvironment, the release of manganese ions from the nanoparticles enhanced the signal intensity on T1-weighted MR images (Fig. 3c).
Tumor blood vessels exhibit structural irregularities, including disorganized and tortuous lumens, varying diameters, and excessive branching, leading to disrupted blood flow, tissue hypoxia, and acidic substance accumulation. This vicious cycle contributes substantially to tumor tissue resistance to RT. However, our designed nanoparticles, which are primarily composed of zinc ions, can alleviate tumor hypoxia by inhibiting complex IV in the mitochondrial ETC, reducing cellular oxidative stress, and decreasing endogenous oxygen consumption21,22,23. Our experimental results support these findings, and upon ameliorating tumor hypoxia, the number of disorganized and enlarged blood vessels within the tumor tissue was reduced (Fig. 5, Fig. 6f–i). Additionally, recent research has revealed that nutritional metal ions such as Mn2+, Zn2+, and Ca2+ can exert immunomodulatory effects akin to those of cytokines24. Building upon this, Zhang et al. developed a Zn-based immune-modulating adjuvant, Zn-LDH, which effectively alleviates immune suppression and stimulates a robust antitumor immune response without requiring additional cytokines or immune stimulants. These novel functionalities of Zn2+ and Mn2+ warrant further research and exploration for potential applications.
In recent years, in situ vaccines have emerged as a novel approach based on old methods, where the tumor itself serves as a vaccine to trigger a systemic antitumor immune response. This concept encompasses various strategies, including RT and intratumoral injection of immunostimulatory agents6,25. RT induces DNA damage and immunogenic cell death, leading to the release of DAMPs such as high mobility group box 1 (HMGB1) and CRT (Fig. 7a)26. These DAMPs act as danger signals, promoting the maturation of DCs and polarization of TAMs toward the M1 phenotype (Fig. 7c–f, Fig. S1)27. Effector T cells activated by these APCs (Fig. 7g–h, Fig. S2) are recruited to the tumor site, where they initiate antitumor immune responses. This process induces apoptosis in residual tumor cells, releasing tumor antigens for recognition by immune cells, thereby enhancing antitumor immunity and achieving the function of RT as an in situ vaccine28. However, the strength of antitumor immunity activated by RT alone is limited, and combining RT with TLR agonists (Fig. 6a–e) and immune checkpoint inhibitors (Fig. 8a–c) can significantly increase its therapeutic efficacy. Nevertheless, the optimal approach for fully exploiting RT as an in situ vaccine remains unclear. Despite the need for further research and exploration, RT remains a cornerstone in cancer treatment and is widely used and well established in clinical practice. Moreover, the reimbursement rates of RT under domestic medical insurance are high, making it economically viable and suitable for widespread application in our country.
For successful clinical translation, ensuring the safety of the MSN@R837-Mn/ZnO2 nanoparticles, in addition to their therapeutic efficacy, is essential. Silica toxicity is mainly attributed to the interaction between silanol groups on the surface and cellular components, leading to cell lysis and death29. However, the mesoporous structure of MSNs results in a lower hemolytic effect than that of nonporous silica, possibly due to the lower silanol density30,31. Studies on in vivo distribution and elimination indicate that MSNs are relatively safe when administered orally or intravenously for biomedical purposes32. The LD50 of MSNs exceeded 1000 mg/kg, and mice treated with low-dose MSNs exhibited no adverse effects33. In our mouse model, the designed nanoparticles did not induce significant liver or kidney damage, nor did they have notable toxic effects on vital organs or on mouse behavior (Fig. 8d-g). Nevertheless, further rigorous experiments are necessary to thoroughly assess the safety of these nanoparticles for potential clinical use.
Conclusions
The main challenges of current RT are the hypoxic tumor environment and the difficulty in eliciting effective antitumor immune responses with RT alone. To address these challenges, we developed a nanodelivery system using MSNs, R837, and Mn/ZnO2, which enables specific accumulation in tumor tissues, controlled drug release in acidic environments, and enhanced T1-weighted MR imaging. The MSN@R837-Mn/ZnO2 nanoparticles significantly improved the therapeutic efficacy of RT by reducing tumor hypoxia and reversing the immunosuppressive tumor microenvironment. Moreover, when combined with PD-1 blockade, the MSN@R837-Mn/ZnO2 nanoparticles further increased the efficacy of RT, leading to effective tumor suppression and prolonged survival. Additionally, safety assessments demonstrated the excellent biocompatibility and absence of toxicity of MSN@R837-Mn/ZnO2, supporting its potential as a promising therapeutic approach for cancer treatment.
References
Huang, R. X. & Zhou, P. K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 5, 60 (2020).
Shaowen, X. & Shanwen, Z. New advances in clinical treatment of radiation oncology. Sci. Technol. Rev. 32, 37–41 (2014).
He, J. et al. Basic guidelines of quality control for radiotherapy. Chin. J. Radiat. Oncol. 27, 335–342 (2018).
Zschaeck, S. et al. Individual patient data meta-analysis of FMISO and FAZA hypoxia PET scans from head and neck cancer patients undergoing definitive radio-chemotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 149, 189–196 (2020).
Brown, J. M. Radiation damage to tumor vasculature initiates a program that promotes tumor recurrences. Int. J. Radiat. Oncol. Biol. Phys. 108, 734–744 (2020).
Golden, E. B., Marciscano, A. E. & Formenti, S. C. Radiation therapy and the in situ vaccination approach. Int. J. Radiat. Oncol. Biol. Phys. 108, 891–898 (2020).
Pierce, R. H., Campbell, J. S., Pai, S. I., Brody, J. D. & Kohrt, H. E. In-situ tumor vaccination: bringing the fight to the tumor. Hum. Vaccin. Immunother. 11, 1901–1909 (2015).
Lurje, I. et al. In situ vaccination as a strategy to modulate the immune microenvironment of hepatocellular carcinoma. Front. Immunol. 12, 650486 (2021).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
He, K., Li, J., Shen, Y. & Yu, Y. pH-Responsive polyelectrolyte coated gadolinium oxide-doped mesoporous silica nanoparticles (Gd(2)O(3)@MSNs) for synergistic drug delivery and magnetic resonance imaging enhancement. J. Mater. Chem. B 7, 6840–6854 (2019).
Teng, Z. et al. Mesoporous silica hollow spheres with ordered radial mesochannels by a spontaneous self-transformation approach. Chem. Mater. 25, 98–105 (2012).
Wang, T. et al. Accuracy of using a patient-derived tumor organoid culture model to predict the response to chemotherapy regimens in stage IV colorectal cancer: a blinded study. Dis. Colon Rectum 64, 833–850 (2021).
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988 e1916 (2018).
Kankala, R. K. et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 32, e1907035 (2020).
Huang, P. et al. Molecularly organic/inorganic hybrid hollow mesoporous organosilica nanocapsules with tumor-specific biodegradability and enhanced chemotherapeutic functionality. Biomaterials 125, 23–37 (2017).
Shao, D. et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 49, 531–540 (2017).
Vallet-Regi, M., Schuth, F., Lozano, D., Colilla, M. & Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: where are we after two decades? Chem. Soc. Rev. 51, 5365–5451 (2022).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557 (2009).
Cauda, V., Argyo, C. & Bein, T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J. Mater. Chem. 20, 8693–8699 (2010).
Sharpley, M. S. & Hirst, J. The inhibition of mitochondrial complex I (NADH:ubiquinone oxidoreductase) by Zn2. J. Biol. Chem. 281, 34803–34809 (2006).
Kuznetsova, S. S., Azarkina, N. V., Vygodina, T. V., Siletsky, S. A. & Konstantinov, A. A. Zinc ions as cytochrome C oxidase inhibitors: two sites of action. Biochemistry (Mosc) 70, 128–136 (2005).
Aagaard, A. & Brzezinski, P. Zinc ions inhibit oxidation of cytochrome c oxidase by oxygen. FEBS Lett. 494, 157–160 (2001).
Wang, C., Zhang, R., Wei, X., Lv, M. & Jiang, Z. Metalloimmunology: the metal ion-controlled immunity. Adv. Immunol. 145, 187–241 (2020).
Bernstein, M. B., Krishnan, S., Hodge, J. W. & Chang, J. Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat. Rev. Clin. Oncol. 13, 516–524 (2016).
Chen, X. et al. Turning up the heat on non-immunoreactive tumors: pyroptosis influences the tumor immune microenvironment in bladder cancer. Oncogene 40, 6381–6393 (2021).
Zhu, M. et al. Immunogenic cell death induction by ionizing radiation. Front. Immunol. 12, 705361 (2021).
Goto, T. Radiation as an in situ auto-vaccination: current perspectives and challenges. Vaccines 7, 100 (2019).
Slowing, I. I., Wu, C. W., Vivero-Escoto, J. L. & Lin, V. S. Mesoporous silica nanoparticles for reducing hemolytic activity towards mammalian red blood cells. Small 5, 57–62 (2009).
Mohammadpour, R. et al. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J. Control. Release 324, 471–481 (2020).
Lin, Y. S. & Haynes, C. L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 132, 4834–4842 (2010).
Kankala, R. K., Han, Y. H., Xia, H. Y., Wang, S. B. & Chen, A. Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnol. 20, 126 (2022).
Narayan, R., Nayak, U. Y., Raichur, A. M. & Garg, S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics 10, 118 (2018).
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 81972309, 81930080, and 82202999], the Fund for Distinguished Young Scholars of Jiangsu Province [grant number BK20230001], the Medical Research Project of Jiangsu Health Commission (No. M2020035) and the Nanjing Jiangbei New Area Key Research and Development Program. We thank all members of the Clinical Cancer Institute of Nanjing University for their discussion and suggestions.
Author information
Authors and Affiliations
Contributions
Conceptualization: Qin Liu, Yanhong Chu, Baorui Liu; Data curation: Yanhong Chu, Lifeng Wang, Yaohua Ke, Xiaoyu Feng, Wenmei Rao; Funding acquisition: Qin Liu, Baorui Liu, Yanhong Chu; Visualization: Wei Ren, Kai Xin, Yan Wang, Lixia Yu; Writing - original draft: Yanhong Chu; Writing - review & editing: Qin Liu, Lifeng Wang. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chu, Y., Wang, L., Ke, Y. et al. A multifunctional mesoporous silica drug delivery nanosystem that ameliorates tumor hypoxia and increases radiotherapy efficacy. NPG Asia Mater 16, 40 (2024). https://doi.org/10.1038/s41427-024-00560-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-024-00560-w
- Springer Japan KK