Abstract
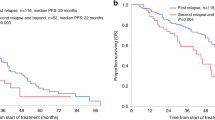
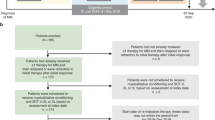
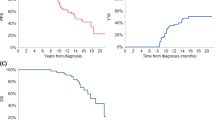
Autologous stem cell transplantation (ASCT) is an integral component of the therapeutic arsenal in multiple myeloma. Given that overall survival (OS) is comparable between patients receiving up-front or delayed ASCT, some opt to collect stem cells and postpone transplant until the time of disease progression (i.e., salvage ASCT). It is unknown if induction should be repeated prior to salvage ASCT, or if patients should proceed directly. We identified 234 patients who underwent salvage ASCT at our institution: 188 (80%) were re-induced, whereas 46 (20%) proceeded directly without re-induction. There was no significant difference in time to next therapy (TNT) or OS from Day 0 between the two groups. Patients who were re-induced had a nonsignificant trend towards a higher rate of complete response post-ASCT (45 vs. 33%, p = .12). In multivariate models, re-induction did not affect TNT/OS. In the subgroup of 188 patients who were re-induced, patients with relapsed/refractory disease at the time of ASCT had significantly shorter TNT/OS compared to patients with deeper pre-ASCT responses. In summary, there was no survival difference for patients who were re-induced before salvage ASCT. However, many factors affect the decision to re-induce, and prospective studies would be required to discern its role definitively.




Similar content being viewed by others
References
Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Prim. 2017;3:17046.
Ravi P, Kumar SK, Cerhan JR, Maurer MJ, Dingli D, Ansell SM, et al. Defining cure in multiple myeloma: a comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018;8:26.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8.
Sidiqi MH, Aljama MA, Bin Riaz I, Dispenzieri A, Muchtar E, Buadi FK, et al. Bortezomib, lenalidomide, and dexamethasone (VRd) followed by autologous stem cell transplant for multiple myeloma. Blood Cancer J. 2018;8:106.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–7.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83.
Mahajan S, Tandon N, Kumar S. The evolution of stem-cell transplantation in multiple myeloma. Ther Adv Hematol. 2018;9:123–33.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Gonsalves WI, Buadi FK, Ailawadhi S, Bergsagel PL, Chanan Khan AA, Dingli, D et al. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement. Bone Marrow Transpl. 2018; https://doi.org/10.1038/s41409-018-0264-8.
D’Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transpl. 2017;23:1417–21.
Shah N, Callander N, Ganguly S, Gul Z, Hamadani M, Costa L, et al. Hematopoietic stem cell transplantation for multiple myeloma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1155–66.
Kumar SK, Buadi FK, Rajkumar SV. Pros and cons of frontline autologous transplant in multiple myeloma: the debate over timing. Blood. 2019;133:652–9.
Dunavin NC, Wei L, Elder P, Phillips GS, Benson DM Jr, Hofmeister CC, et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma. 2013;54:1658–64.
Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–92.
Richardson PG, Laubach JP, Munshi NC, Anderson KC. Early or delayed transplantation for multiple myeloma in the era of novel therapy: does one size fit all? Hematol Am Soc Hematol Educ Program. 2014;2014:255–61.
Sidana S, Tandon N, Dispenzieri A, Gertz MA, Buadi FK, Lacy MQ, et al. Relapse after complete response in newly diagnosed multiple myeloma: implications of duration of response and patterns of relapse. Leukemia. 2019;33:730–8.
Lahuerta JJ, Paiva B, Vidriales MB, Cordón L, Cedena MT, Puig N, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–10.
Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013;31:4529–35.
Vij R, Kumar S, Zhang MJ, Zhong X, Huang J, Dispenzieri A, et al. Impact of pretransplant therapy and depth of disease response before autologous transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2015;21:335–41.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57.
Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20.
Dispenzieri A, Kyle R, Merlini G, Miguel J, Ludwig H, Hajek R, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–24.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5.
Rajkumar SV, Richardson P, San Miguel JF. Guidelines for determination of the number of prior lines of therapy in multiple myeloma. Blood. 2015;126:921–2.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
van Rhee F, Giralt S, Barlogie B. The future of autologous stem cell transplantation in myeloma. Blood. 2014;124:328–33.
Shah GL, Winn AN, Lin P-J, Klein A, Sprague KA, Smith HP, et al. Cost-effectiveness of autologous hematopoietic stem cell transplantation for elderly patients with multiple myeloma using the surveillance, epidemiology, and end results-medicare database. Biol Blood Marrow Transplant. 2015;21:1823–9.
Paiva B, Vídriales MB, Rosiñol L, Martínez-López J, Mateos MV, Ocio EM, et al. A multiparameter flow cytometry immunophenotypic algorithm for the identification of newly diagnosed symptomatic myeloma with an MGUS-like signature and long-term disease control. Leukemia. 2013;27:2056–61.
Kansagra A, Gonsalves WI, Gertz MA, Buadi FK, Dingli D, Dispenzieri A, et al. Analysis of clinical factors and outcomes associated with nonuse of collected peripheral blood stem cells for autologous stem cell transplants in transplant-eligible patients with multiple myeloma. Biol Blood Marrow Transplant. 2018;24:2127–32.
Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–7.
Sellner L, Heiss C, Benner A, Raab MS, Hillengass J, Hose D, et al. Autologous retransplantation for patients with recurrent multiple myeloma: a single-center experience with 200 patients. Cancer. 2013;119:2438–46.
Gonsalves WI, Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Second auto-SCT for treatment of relapsed multiple myeloma. Bone Marrow Transplant. 2013;48:568–73.
Acknowledgements
We would like to acknowledge the patients and their families. KM was supported by CTSA Grant Number TL1TR002380 from the National Center for Advancing Translational Sciences (NCATS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SK has obtained research support for clinical trials from Celgene, Millennium, Novartis, Janssen, and Sanofi. AD has received research support for clinical trials from Pfizer, Jannsen, Millennium, Alnylam, and Celgene. MAG has received research support from ISIS and Prothena, and honoraria from Celgene, Millennium Pharmaceuticals, and Novartis. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Parts of these data were presented on Poster 4613 at the American Society of Hematology Annual Meeting in San Diego, California on 03 December 2018.
Rights and permissions
About this article
Cite this article
Miller, K.C., Gertz, M.A., Buadi, F.K. et al. The impact of re-induction prior to salvage autologous stem cell transplantation in multiple myeloma. Bone Marrow Transplant 54, 2039–2050 (2019). https://doi.org/10.1038/s41409-019-0590-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0590-5
- Springer Nature Limited
This article is cited by
-
Carfilzomib, lenalidomide and dexamethasone followed by a second ASCT is an effective strategy in first relapse multiple myeloma: a study on behalf of the Chronic malignancies working party of the EBMT
Bone Marrow Transplantation (2023)
-
Depth of response prior to autologous stem cell transplantation predicts survival in light chain amyloidosis
Bone Marrow Transplantation (2021)
-
Outcomes after delayed and second autologous stem cell transplant in patients with relapsed multiple myeloma
Bone Marrow Transplantation (2021)