Abstract
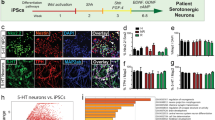
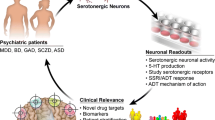
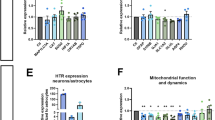
Selective serotonin reuptake inhibitors (SSRIs) are the most prescribed antidepressants. They regulate serotonergic neurotransmission, but it remains unclear how altered serotonergic neurotransmission may contribute to the SSRI resistance observed in approximately 30% of major depressive disorder (MDD) patients. Patient stratification based on pharmacological responsiveness and the use of patient-derived neurons may make possible the discovery of disease-relevant neural phenotypes. In our study from a large cohort of well-characterized MDD patients, we have generated induced pluripotent stem cells (iPSCs) from SSRI-remitters and SSRI-nonremitters. We studied serotonergic neurotransmission in patient forebrain neurons in vitro and observed that nonremitter patient-derived neurons displayed serotonin-induced hyperactivity downstream of upregulated excitatory serotonergic receptors, in contrast to what is seen in healthy and remitter patient-derived neurons. Our data suggest that postsynaptic forebrain hyperactivity downstream of SSRI treatment may play a role in SSRI resistance in MDD.





Similar content being viewed by others
References
http://www.who.int/mediacentre/factsheets/fs369/en/, http://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml, https://www.nlm.nih.gov/medlineplus/ency/article/000945.htm & http://www.dbsalliance.org/site/PageServer?pagename=education_statistics_depression
Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3:171–8.
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–38.
Charney DS, Buxbaum JD, Sklar P, Nestler EJ. Neurobiology of mental illness. Oxford University Press: 2013.
Breen G, et al. Translating genome-wide association findings into new therapeutics for psychiatry. Nat Neurosci. 2016;19:1392–6.
Levinson DF, et al. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol Psychiatry. 2014;76:510–2.
Wray NR et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81. https://doi.org/10.1038/s41588-018-0090-3
Soliman MA, Aboharb F, Zeltner N, Studer L. Pluripotent stem cells in neuropsychiatric disorders. Mol Psychiatry. 2017;22:1241–9.
Brennand KJ, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–5.
Robicsek O, et al. Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatry. 2013;18:1067–76.
Madison JM, et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry. 2015;20:703–17.
Mertens J, et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527:95–9.
Mrazek DA, et al. Treatment outcomes of depression: the pharmacogenomic research network antidepressant medication pharmacogenomic study. J Clin Psychopharmacol. 2014;34:313–7.
Drysdale AT, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Woo YS, Wang HR, Bahk WM. Lurasidone as a potential therapy for bipolar disorder. Neuropsychiatr Dis Treat. 2013;9:1521–9.
Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24.
McGrath CL, et al. Pretreatment brain states identify likely nonresponse to standard treatments for depression. Biol Psychiatry. 2014;76:527–35.
Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60.
Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216.
Artigas F. Serotonin receptors involved in antidepressant effects. Pharmacol Ther. 2013;137:119–31.
Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500.
Hrdina PD, Du L. Levels of serotonin receptor 2A higher in suicide victims? Am J Psychiatry. 2001;158:147–8.
Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249–64.
Knight AR, et al. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiede Arch Pharmacol. 2004;370:114–23.
Cusack B, Nelson A, Richelson E. Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994;114:559–65.
Benekareddy M, Vadodaria KC, Nair AR, Vaidya VA. Postnatal serotonin type 2 receptor blockade prevents the emergence of anxiety behavior, dysregulated stress-induced immediate early gene responses, and specific transcriptional changes that arise following early life stress. Biol Psychiatry. 2011;70:1024–32.
Marek GJ, Carpenter LL, McDougle CJ, Price LH. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology. 2003;28:402–12.
Sarkisyan G, Roberts AJ, Hedlund PB. The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav Brain Res. 2010;209:99–108.
Mullins UL, Gianutsos G, Eison AS. Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology. 1999;21:352–67.
Sowa-Kucma M, et al. Vortioxetine: a review of the pharmacology and clinical profile of the novel antidepressant. Pharmacol Rep. 2017;69:595–601.
Vadodaria KC, Amatya DN, Marchetto MC, Gage FH. Modeling psychiatric disorders using patient stem cell-derived neurons: a way forward. Genome Med. 2018;10:1.
Rush AJ, et al. An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry. 2006;59:493–501.
Marchetto MC, et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry. 2017;22:820–35.
Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106.
Santos R, et al. Differentiation of inflammation-responsive astrocytes from glial progenitors generated from human induced pluripotent stem cells. Stem Cell Rep. 2017;8:1757–69.
Acknowledgements
This research was supported by Robert and Mary Jane Engman Foundation, Lynn and Edward Streim, Takeda-Sanford Consortium Innovation Alliance grant program (Takeda Pharmaceutical Company). KCV was supported by the Swiss National Science Foundation (SNSF) outgoing postdoctoral fellowship. Salk core facilities are supported by the Cancer center (NCI P30 CA014195). Patient enrollment and iPSC generation were funded by Minnesota Partnership Award for Biotechnology and Medical Genomics (YJ) and the 2012 Mayo Clinic Center for Regenerative Medicine (YJ). YJ was supported by the NIH-Mayo Clinic KL2 Mentored Career Development Award (NCAT UL1TR000135) and the Gerstner Family Mayo Career Development Award in Individualized Medicine. Patient recruitment and the laboratory aspects of the clinical trial were funded by NIH U19 GM61388 (PGRN) and NIH RO1 GM28157. The authors would also like to acknowledge the staff and investigators of the PGRN-AMPS study for their contributions, particularly the late Dr. David A. Mrazek, the former Principal Investigator of the PGRN-AMPS study within the Mayo Clinic NIH-PGRN (U19 GM61388). This research would not have been possible without Dr. Mrazek’s pioneering vision and dedication to antidepressant pharmacogenomics research. We also thank Dr. Manching Ku for help with RNA sequencing, Galina Erikson for help with sequencing data, and ML Gage for editorial comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vadodaria, K.C., Ji, Y., Skime, M. et al. Serotonin-induced hyperactivity in SSRI-resistant major depressive disorder patient-derived neurons. Mol Psychiatry 24, 795–807 (2019). https://doi.org/10.1038/s41380-019-0363-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0363-y
- Springer Nature Limited
This article is cited by
-
Challenges and opportunities for discovering the biology of rare genetic diseases of the brain
Journal of Biosciences (2024)
-
Monozygotic twins discordant for schizophrenia differ in maturation and synaptic transmission
Molecular Psychiatry (2024)
-
Advancing preclinical models of psychiatric disorders with human brain organoid cultures
Molecular Psychiatry (2023)
-
Integrating genetics and transcriptomics to study major depressive disorder: a conceptual framework, bioinformatic approaches, and recent findings
Translational Psychiatry (2023)
-
Molecular pathways of major depressive disorder converge on the synapse
Molecular Psychiatry (2023)