Abstract
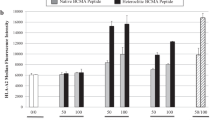
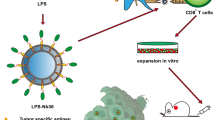
The purpose of these studies was to develop and characterize B-cell maturation antigen (BCMA)-specific peptide-encapsulated nanoparticle formulations to efficiently evoke BCMA-specific CD8+ cytotoxic T lymphocytes (CTL) with poly-functional immune activities against multiple myeloma (MM). Heteroclitic BCMA72−80 [YLMFLLRKI] peptide-encapsulated liposome or poly(lactic-co-glycolic acid) (PLGA) nanoparticles displayed uniform size distribution and increased peptide delivery to human dendritic cells, which enhanced induction of BCMA-specific CTL. Distinct from liposome-based nanoparticles, PLGA-based nanoparticles demonstrated a gradual increase in peptide uptake by antigen-presenting cells, and induced BCMA-specific CTL with higher anti-tumor activities (CD107a degranulation, CTL proliferation, and IFN-γ/IL-2/TNF-α production) against primary CD138+ tumor cells and MM cell lines. The improved functional activities were associated with increased Tetramer+/CD45RO+ memory CTL, CD28 upregulation on Tetramer+ CTL, and longer maintenance of central memory (CCR7+ CD45RO+) CTL, with the highest anti-MM activity and less differentiation into effector memory (CCR7− CD45RO+) CTL. These results provide the framework for therapeutic application of PLGA-based BCMA immunogenic peptide delivery system, rather than free peptide, to enhance the induction of BCMA-specific CTL with poly-functional Th1-specific anti-MM activities. These results demonstrate the potential clinical utility of PLGA nanotechnology-based cancer vaccine to enhance BCMA-targeted immunotherapy against myeloma.







Similar content being viewed by others
Change history
16 January 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41375-020-0705-4
References
Miliotou AN, Papadopoulou LC.CAR T-cell therapy: a new era in cancer immunotherapy.Curr Pharm Biotechnol. 2018;19:5–18.
Legut M, Sewell AK. Designer T-cells and T-cell receptors for customized cancer immunotherapies. Curr Opin Pharm. 2018;41:96–103.
Ping Y, Liu C, Zhang Y. T-cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell. 2018;9:254–66.
Salter AI, Pont MJ, Riddell SR. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood. 2018;131:2621–9.
Curran MA, Glisson BS. New hope for therapeutic cancer vaccines in the era of immune checkpoint modulation. Annu Rev Med. 2018. https://doi.org/10.1146/annurev-med-050217-121900.
Chamani R, Ranji P, Hadji M, Nahvijou A, Esmati E, Alizadeh AM. Application of E75 peptide vaccine in breast cancer patients: a systematic review and meta-analysis. Eur J Pharm. 2018;831:87–93.
Bae J, Carrasco R, Lee AH, Prabhala R, Tai YT, Anderson KC, et al. Identification of novel myeloma-specific XBP1 peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma. Leukemia. 2011;25:1610–9.
Bae J, Tai YT, Anderson KC, Munshi NC. Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders. Br J Haematol. 2011;155:349–61.
Bae J, Song W, Smith R, Daley J, Tai YT, Anderson KC, et al. A novel immunogenic CS1-specific peptide inducing antigen-specific cytotoxic T lymphocytes targeting multiple myeloma. Br J Haematol. 2012;157:687–701.
Nooka AJ, Wang M, Yee AJ, Kaufman J, Bae J, Peterkin D, et al. Safety and Immunogenicity of PVX-410 Vaccine ± lenalidomide in smoldering multiple myeloma. JAMA Oncol. 2018;4:e183267. https://doi.org/10.1001/jamaoncol.2018.3267.
Tran T, Blanc C, Granier C, Saldmann A, Tanchot C, Tartour E. Therapeutic cancer vaccine: building the future from lessons of the past. Semin Immunopathol. 2018. https://doi.org/10.1007/s00281-018-0691-z.
Scharping NE, Delgoffe GM. Tumor microenvironment metabolism: a new checkpoint for anti-tumor immunity. Vaccinnes (Basel). 2016;4:E46.
Strauss J, Madan RA, Gulley JL. Considerations for the combination of anticancer vaccines and immune checkpoint inhibitors. Expert Opin Biol Ther. 2016;16:895–901.
Moreaux J, Legouffe E, Jourdan E, Quittet P, Re’me T, Lugagne C, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–57.
O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–8.
Coquery CM, Erickson LD. Regulatory roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol. 2012;32:287–305.
Bae J, Samur M, Richardson P, Munshi NC, Anderson KC. Selective targeting of multiple myeloma by B cell maturation antigen (BCMA)-specific central memory CD8+ cytotoxic T lymphocytes: immunotherapeutic application in vaccination and adoptive immunotherapy. Leukemia. 2019. https://doi.org/10.1038/s41375-019-0414-z. [Epub ahead of print].
Sahoo SK, Ma W, Labhasetwar V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int J Cancer. 2004;112:335–40.
Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2018;15:31–46.
O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:eaaa0984.
Parvizpour Sepideh, Razmara Jafar, Omidi Yadollah. Breast cancer vaccination comes to age: impacts of bioinformatics. Bioimpacts. 2018;8:223–35.
Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res. 2013;19:4801–15.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62.
Kenderian SS, Porter DL, Gill S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: how not to put the CART before the horse. Biol Blood Marrow Transpl. 2017;23:235–46.
Schwarzbich MA, Witzens-Harig M. Cellular immunotherapy in B-cell malignancy. Oncol Res Treat. 2017;40:674–81.
Buddolla AL, Kim S. Recent insights into the development of nucleic acid-based nanoparticless for tumor-targeted drug delivery. Colloids Surf B Biointerfaces. 2018;172:315–22.
Deshantri AK, Varela Moreira A, Ecker V, Mandhane SN, Schiffelers RM, Buchner M, et al. Nanomedicines for the treatment of hematological malignancies. J Control Release. 2018;287:194–215.
Iqbal J, Abbasi BA, Ahmad R, Mahmood T, Ali B, Khalil AT, et al. Nanomedicines for developing cancer nanotherapeutics: from benchtop to bedside and beyond. Appl Microbiol Biotechnol. 2018;102:9449–70.
Tabassum N, Verma V, Kumar M, Kumar A, Singh B. Nanomedicine in cancer stem cell therapy: from fringe to forefront. Cell Tissue Res. 2018. https://doi.org/10.1007/s00441-018-2928-5.
Zhang Y, Zhang P, Zhu T. Ovarian carcinoma biological nanotherapy: comparison of the advantages and drawbacks of lipid, polymeric, and hybrid nanoparticles for cisplatin delivery. Biomed Pharm. 2018;109:475–83.
Desfrançois C, Auzély R, Texier I. Lipid nanoparticles and their hydrogel composites for drug delivery: a review. Pharmaceuticals (Basel). 2018;11:E118. https://doi.org/10.3390/ph11040118.
Hong SJ, Ahn MH, Lee YW, Pal S, Sangshetti J, Arote RB. Biodegradable polymeric nanocarrier-based immunotherapy in hepatitis vaccination. Adv Exp Med Biol. 2018;1078:303–20.
Aftab S, Shah A, Nadhman A, Kurbanoglu S, Aysıl Ozkan S, Dionysiou DD, et al. Nanomedicine: an effective tool in cancer therapy. Int J Pharm. 2018;540:132–49.
Aw MS, Paniwnyk L. Overcoming T. gondii infection and intracellular protein nanocapsules as biomaterials for ultrasonically controlled drug release. Biomater Sci. 2017;5:1944–61.
Bayford R, Rademacher T, Roitt I, Wang SX. Emerging applications of nanotechnology for diagnosis and therapy of disease: a review. Physiol Meas. 2017;38:R183–R203.
Tang Q, Yu B, Gao L, Cong H, Song N, Lu C. Stimuli responsive nanoparticles for controlled anti-cancer drug release. Curr Med Chem. 2018;25:1837–66.
Jahan ST, Sadat SM, Haddadi A. Design and immunological evaluation of anti-CD205-tailored PLGA-based nanoparticulate cancer vaccine. Int J Nanomed. 2018;13:367–86.
Kim H, Niu L, Larson P, Kucaba TA, Murphy KA, James BR, et al. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials. 2018;164:38–53.
Wang D, Sun Y, Liu Y, Meng F, Lee RJ. Clinical translation of immunoliposomes for cancer therapy: recent perspectives. Expert Opin Drug Deliv. 2018;15:893–903.
Graziani SR, Vital CG, Morikawa AT, Van Eyll BM, Fernandes Junior HJ, Kalil Filho R, et al. Phase II study of paclitaxel associated with lipid core nanoparticles (LDE) as third-line treatment of patients with epithelial ovarian carcinoma. Med Oncol. 2017;34:151.
Grabbe S, Haas H, Diken M, Kranz LM, Langguth P, Sahin U. Translating nanoparticulate-personalized cancer vaccines into clinical applications: case study with RNA-lipoplexes for the treatment of melanoma. Nanomedicine (Lond). 2016;11:2723–34.
Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomized, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68.
Vansteenkiste JF, Vanakesa T, De Pas T, Zielinski M, Kim MS, Jassem J, et al. MAGRIT, a double-blind, randomized, placebo-controlled Phase III study to assess the efficacy of the RecMAGE-A3 + AS15 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-Positive non-small cell lung cancer (NSCLC). Ann Oncol. 2014;25:409–16.
Kruit WHJ, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organization for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol. 2013;31:2413–20.
Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:822–35.
Limentani SA, Campone M, Dorval T, Curigliano G, de Boer R, Vogel C, et al. A non-randomized dose-escalation Phase I trial of a protein-based immunotherapeutic for the treatment of breast cancer patients with HER2-overexpressing tumors. Breast Cancer Res Treat. 2016;156:319–30.
Berinstein NL, Karkada M, Oza AM, Odunsi K, Villella JA. Nemunai- tis JJ et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology. 2015;4:e1026529.
Saito T, Wada H, Yamasaki M, Miyata H, Nishikawa H, Sato E, et al. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine. 2014;32:5901–7.
Bavananthasivam J, Alkie TN, Astill J, Abdul-Careem MF, Wootton SK, Behboudi S, et al. In ovo administration of Toll-like receptor ligands encapsulated in PLGA nanoparticles impede tumor development in chickens infected with Marek’s disease virus. Vaccine. 2018;36:4070–6.
Thompson EA, Ols S, Miura K, Rausch K, Narum DL, Spångberg M, et al. TLR-adjuvanted nanoparticles vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight. 2018;3:120692.
Zupančič E, Curato C, Paisana M, Rodrigues C, Porat Z, Viana AS, et al. Rational design of nanoparticles towards targeting antigen-presenting cells and improved T cell priming. J Control Release. 2017;258:182–95.
Salvador A, Igartua M, Hernandez RM, Pedraz JL. Combination of immune stimulating adjuvants with poly(lactide-co-glycolide) microspheres enhances the immune response of vaccines. Vaccine. 2012;30:589–96.
Lee YR, Lee YH, Im SA, Kim K, Lee CK. Formulation and characterization of antigen-loaded PLGA nanoparticles for efficient cross-priming of the antigen. Immune Netw. 2011;11:163–8.
Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–7.
Allahyari M, Mohit E. Peptide/protein vaccine delivery system based on PLGA particles. Hum Vaccin Immunother. 2016;12:806–28.
Silva AL, Rosalia RA, Varypataki E, Sibuea S, Ossendorp F, Jiskoot W. Poly-(lactic-co-glycolic-acid)-based particulate vaccines: particle uptake by dendritic cells is a key parameter for immune activation. Vaccine. 2015;33:847–54.
Saini V, Jain V, Sudheesh MS, Jaganathan KS, Murthy PK, Kohli DV. Comparison of humoral and cell-mediated immune responses to cationic PLGA microspheres containing recombinant hepatitis B antigen. Int J Pharm. 2011;408:50–7.
Feng L, Qi XR, Zhou XJ, Maitani Y, Wang SC, Jiang Y, et al. Pharmaceutical and immunological evaluation of a single-dose hepatitis B vaccine using PLGA microspheres. J Control Release. 2006;112:35–42.
Jaganathan KS, Singh P, Prabakaran D, Mishra V, Vyas SP. Development of a single-dose stabilized poly(D,L-lactic-co-glycolic acid) microspheres-based vaccine against hepatitis B. J Pharm Pharm. 2004;56:1243–50.
Rosas JE, Pedraz JL, Hernandez RM, Gascon AR, Igartua M, Guz- man F, et al. Remarkably high antibody levels and protection against P. falciparum malaria in Aotus monkeys after a single immunisation of SPf66 encapsulated in PLGA microspheres. Vaccine. 2002;20:1707–10.
Acknowledgements
The authors acknowledge the Miriam and Sheldon G. Adelson Medical Research Foundation. This work was supported in part by grants from the National Institutes of Health Grants Special Program in Oncology Research Excellence (SPORE) P50 100707, RO1 CA 207237, and RO1 CA 050947. Dr. Kenneth C. Anderson is an American Cancer Society Clinical Research Professor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bae, J., Parayath, N., Ma, W. et al. BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8+ cytotoxic T lymphocytes against multiple myeloma: clinical applications. Leukemia 34, 210–223 (2020). https://doi.org/10.1038/s41375-019-0540-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0540-7
- Springer Nature Limited
This article is cited by
-
Drug delivery methods for cancer immunotherapy
Drug Delivery and Translational Research (2024)
-
Development and application of nanomaterials, nanotechnology and nanomedicine for treating hematological malignancies
Journal of Hematology & Oncology (2023)
-
Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation
Molecular Cancer (2023)
-
Advancement and Applications of Nanotherapy for Cancer Immune Microenvironment
Current Medical Science (2023)
-
Mass spectrometry-based identification of a B-cell maturation antigen-derived T-cell epitope for antigen-specific immunotherapy of multiple myeloma
Blood Cancer Journal (2020)