Abstract
Freeze-drying (FD), or lyophilization, is commonly used to preserve foods. FD offers potential to create a human milk-derived human milk fortifier, and an alternative to freeze-storing human milk. However, processing human milk is known to affect its components. This scoping review explores the effect of FD on the; macronutrient, micronutrient, vitamin, bioactive components, microbes and anti-microbial factors in human milk, and studies where lyophilized human milk has been given to newborn infants. 48 articles were identified after full text review. FD human milk reduces the fat globule size and as well as the quantity of enzymes, vitamin C and immunoglobulin. Common serum electrolyte disturbances have been reported when preterm infants’ are fed FD human milk however it appears a promising method to avoid exposure of preterm infants’ to cows’ milk. Due to limited data, further studies exploring the safety and efficacy of FD human milk in preterm infants are needed.
Similar content being viewed by others
Introduction
Freeze-drying (FD), or lyophilization, has been long described for preserving food products [1]. FD involves freezing a substance and then using low pressure to sublime the frozen water into vapor, leaving a dry product [2] The amount of water extracted during the process can be adjusted [1].
FD human milk as a concept is appealing to organizations who care for newborn infants; both as a possible means to reduce storage challenges of human milk and as an additive to human milk (fortification) in order to offer additional nutrients to preterm infants (commonly met by fortifiers derived from bovine milk). Human milk is the preferred diet for newborn infants for multiple reasons including associations with improved long-term maternal and infant health and reduced major neonatal morbidities, such as necrotizing enterocolitis and bronchopulmonary dysplasia [3,4,5]. However, human milk alone does not meet the high nutritional needs of preterm infants. Concentrations of protein, sodium, calcium and phosphorous often do not meet the needs of preterm infants [6].
There remain concerns regarding the effect that FD could have on what is a complex biological fluid. Alterations to human milk could lead to excessive or inadequate macronutrients, micronutrients or vitamins. FD could lead to bacteria growth or loss of anti-microbials and bioactive components. Furthermore, there could be functional changes to any of these elements.
The aim of this review is to examine the effect of FD on the nutrients and bioactive composition of human milk and review the clinical effects of feeding FD human milk to preterm infants.
Methods
We followed the guidelines of PRISMA.
Inclusion criteria
We included pre-clinical and clinical studies. Studies published in non-English language or as abstracts were not included. The search was limited to human milk only. Criteria for inclusion and exclusion clinical studies were based on PICOD format—(1) target population: preterm infants; (2) intervention: any FD supplement; (3) comparison: bovine-based supplementation or no intervention; (4) outcome: growth as defined in each study or any other changes to milk content in pre-clinical studies; and (5) study design: randomized-controlled and quasi-randomized trials. Search was performed electronically. All retrieved articles were reviewed to determine studies that comprised preterm infants. The differences in opinion encountered during the process of review were resolved by consensus.
Search strategy
The literature search was performed in December 2022. Medline, Pubmed, Scopus, Web of Science, Cochrane Library and Embase were searched individually with the following terms [“freeze dried” OR “freeze dry*” OR “lyophili*” OR “freez*” OR “Freeze Drying” OR “frozen”] AND [“human milk” OR “breast milk”]. All publication types from 1980-2022 were then reviewed electronically using a systematic review management tool (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). We used 1980 as a cut-off as there were multiple steps to standardize the FD process which were undertaken from 1980 [7]. The combination of text words and exploded medical subject headings were used to maximize the quantity of the data and articles retrieved.
Two study members screened each abstract. Any abstract that was screened as potentially eligible then had a full text review by two study members. Articles were deemed eligible if they described the effect of FD on either the composition of human milk, or clinical effect of FD human milk in newborn infants. Articles were excluded if they were not readily available in English, did not provide new data or offer a comparator.
Data extraction and synthesis
Data charting was completed independently in a standardized format as described by Arksey and O’Malley [8]. Data items sought were year of publication, country of publication, type of milk used (mothers own milk (MOM) or donor human milk (DHM), MOM was defined as freshly expressed milk for analysis without freezing or pasteurization), participants (mothers and/or infants), sample size, setting (in vitro, in vivo), comparison used, FD process (device, pressure, temperature (freezing and for heating), length), and outcomes measured.
Results
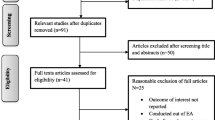
We identified 2073 studies using our selection criteria with two additional articles discovered on citation searching. After abstract review and removal of duplicates, 63 full texts remained. Forty-eight articles remained after full text review (Fig. 1). Articles covered all areas of lyophilization of human milk which we have separated into the effect of FD on the macronutrient, micronutrient & vitamins, bioactive component and microbial/anti-microbial composition of human milk. We have separately described articles exploring the clinical effect of feeding FD human milk to newborn infants. The results are presented based on the primary focus of each study (Tables 1–5). Figure 2 summarizes the key study findings.
Macronutrients
Fourteen articles were identified that describe the effect of FD on the macronutrient composition of human milk (Table 1). Two studies described using MOM whilst the others reported using donor human milk (DHM). The study design varied with 4 studies exploring the macronutrient composition of human milk fortified with FD human milk, and the remaining 10 studies exploring the effect of FD on human milk composition.
Lipids
Two studies report that the total lipid content of human milk does not reduce following FD [9, 10]. However, both an increased concentration of lipids in FD DHM (compared to raw DHM) [11, 12], and an assumed decreased concentration of fats (concentration of fat did not double when adding FD DHM to DHM suggesting a loss in fats) have been reported [13]. Several studies explored the effect of FD on the free fatty acid (FFA) composition or lipid profiles of human milk. The majority of these studies did not report any significant change in either FFA composition or lipid profiles over time [10, 11, 14, 15]. However, Blackshaw et al. did report an increase in the total FFA after FD [16]. They postulated that this could be related to preserved lipase activity or to damage or structural changes to the milk fat globule [16]. FD has been shown to significantly decrease the human milk fat globule size, therefore increasing its surface area which may improve its bioavailability [9].
Storage of FD human milk, for up to 4 months at a temperature of 4 °C (or less) has not been reported to change the composition of FFA [10, 17]. However, storage at 25°C or prolonged beyond 120 days led to accumulation of total FFA and monounsaturated fatty acids, respectively [10, 12, 17].
Protein
Five studies have explored the effect of FD on the protein content of human milk. All of these studies reported minimal total protein loss in human milk following FD [12, 13, 18,19,20]. Furthermore, there has been shown to be no difference in total protein content with up to 6 months storage (4 °C or –80 °C) of FD human milk [18] or in comparison to freezing human milk [20, 21]. FD fortified human milk has been shown to have an increased protein content in comparison to human milk fortified with cows’ milk fortifier (CMF) and human milk fortified with evaporated human milk, however within recommended nutritional intakes [22]. Whilst total protein content does not seem to be affected by FD we have explored the effect of FD on specific proteins in the bioactive components section below.
Carbohydrate
The main carbohydrate in human milk is lactose. A lactose removal step before FD is often reported, making it difficult to interpret any effect of FD on carbohydrate content [19, 22, 23].
Variation in the lactose removal step may explain differences in the reported carbohydrate content of FD human milk. Thomaz et al. report less carbohydrate (7.25 ± 0.25 g/dl) in FD human milk combined with DHM than Grance et al. (9.22 ± 1 g/dl) despite both studies involving a lactose removal step [19, 22]. Human milk oligosaccharides (HMO’s) are the other major human milk carbohydrate, with their composition not being affected by FD [24].
The evidence regarding whether FD human milk can be used as a human milk fortifier to achieve recommended macronutrients intakes for preterm infants’ is unclear. Grance et al reported that the recommended (European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN)) macronutrient intake could be achieved by using FD DHM combined with DHM [19]. However, Fusch et al. report that there is no ideal lyophilization factor that leads to recommended (ESPGHAN) macronutrient intake when using FD MOM as a human milk fortifier [25]. Fusch et al. report that a lyophilization factor of 1.2 will lead to an acceptable fat content, however deficient protein, carbohydrate and energy content. Their results suggest that any increase in the lyophilization factor will lead to excessive macronutrients [25]. The different results of these studies could be related to variation in the processing (lactose and fat removal, pasteurization) of human milk, or to the use of DHM and MOM.
Osmolality
Osmolality is a measure of the number of solute particles in a solvent, whilst osmolarity is a measure of the number of solute particles in a solution.
Three studies have reported inconsistent results on the osmolality of FD fortified human milk as you would expect higher osmolality with increasing concentration of FD HM. Oliveira et al reported an osmolality of 452 mOsm/kg when combining 50 ml FD HM with 75 ml DHM [12]. The osmolality increased to 456 mOsm/kg following 6 months storage [12]. Grance reported an osmolality of 389.6 mOSm/kg when combining 45 ml FD DHM with 50 ml DHM [19]. Grance et al and Oliveira et al did report the mean osmolality of the human milk before addition of FD HM which was 22 mOSm/kg higher in the study by Oliveira et al which may explain some the discrepancy in the results likely due to variation in DHM [12, 19].
Micronutrients and vitamins
There have been concerns that FD would increase the concentration of micronutrients and vitamins to excessive levels. We identified six studies that explored the effect of FD on the micronutrient composition of human milk, and three studies that explored the effect of FD on vitamin composition.
Micronutrients
The micronutrient concentration of human milk fortified with FD human milk has been compared to the baseline micronutrient concentration of human milk. Using this comparison, there has been shown to be an increase in most micronutrients namely; calcium, phosphorous [12, 13, 19, 22], magnesium [12, 13], sodium, potassium [12, 19], copper, zinc [12], manganese and selenium [26], however not to excessive levels. An outlier was the study by Lucas et al. which did not find an increase in sodium and potassium concentrations, potentially related to the FD method used in this study [13].
Comparing human milk fortified with FD human milk to human milk alone, there was no significant change in the concentration of potentially toxic elements (aluminum, arsenic, cadmium, chromium, mercury, iron, nickel, tin, thallium) [26]. This study did report a significant decrease in the concentration of lead [26]. Furthermore, lead and nickel concentrations do not seem to be affected by the geographical location or socio-economic status of the mother although due to changing diets and environmental exposures the reported concentration of these elements may not be representative of current human milk [27].
Lastly, it should be noted that human milk fortified with FD human milk has significantly less calcium and phosphorous than human milk fortified with CMF [22]. A diet using donor human milk fortified with FD human milk has been shown to meet the sodium and potassium requirements of preterm infants, but not the calcium or phosphorous requirements as defined by ESPGHAN [22].
Vitamins
Three studies have reported the effect of FD on the vitamin concentration of human milk. Whilst there appears to be no effect on the concentrations of B vitamins (Niacin, biotin, pantothenic acid) [15], a 31.5% reduction in vitamin C concentration has been reported following FD [28]. Interestingly, storage of FD human milk leads to increased retention of vitamin C (total and ascorbic acid) and vitamin E in comparison to human milk stored fresh or frozen [29].
Bioactive components
Human milk is a complicated biological fluid that contains a huge number of molecules that influence the developing immune system [30, 31]. Collectively these molecules have been termed bioactive components and include enzymes, immunoglobulin, immune cells (e.g. lymphocytes, stem cells), oligosaccharides, hormones and cytokines.
FD affects bioactive components to varying degrees. Human milk contains specific glycoproteins felt to be important for lipid breakdown and immune development. The glycoproteins, bile-salt stimulated lipase and esterase, have both been shown to reduce in quantity following FD and storage at –20 °C [32,33,34]. Similarly, FD leads to loss of approximately 20% of Immunoglobulin (Ig) G and IgM, whereas IgA appears more sensitive with greater losses which may be reduced depending on the heating plate temperature or holder pasteurization (HoP) temperature, if this is used in milk processing [16, 35, 36]. Whilst leptin, adiponectin, lysozyme and lactoferrin are not affected by FD [32, 37, 38], storage has been shown to increase the activity of lysozyme [38] and HoP decrease the concentration of leptin [32].
Human milk growth factors are thought to be important for the maturation and development of all organ systems [39]. Whilst FD alone has no impact on hepatocyte growth factor or insulin concentration, HoP then FD leads to a significant reduction in hepatocyte growth factor concentration [32].
FD does not affect the composition of human milk oligosaccharides (HMO’s) which are important for gut microbial development and immune maturation
Anti-oxidant capacity is thought to be an important component of human milk especially in preterm infants [39]. FD does not appear to affect a range of human milk anti-oxidants including polyphenols, lipoperoxides, hydroperoxides, superoxide anion, or nitrites [18, 38, 40]. However, there is varying reports of the impact of storing FD milk with one study reporting a 20% reduction in total anti-oxidant capacity, which was exacerbated by higher storage temperatures [38], whilst a similar study reported no change to several antioxidants after 6 months of storage [18].
Human milk cytokines may be important for modulating any intestinal inflammatory response. Cytokine levels are not altered by FD [40].
Microbial and anti-microbial factors
Human milk contains bacteria that are beneficial for infants as well as factors that inhibit the growth of pathogenic bacteria.
FD has been shown to decrease the bacterial count of human milk, whilst the acidity (reflection of contamination) has been shown to remain unchanged [10, 41]. These studies did not explore the specific bacteria affected. FD significantly reduces the concentration of Staphylococcus epidermidis and other potentially pathogenic organisms (mesophilic aerobic microorganisms) in comparison to freezing milk [42]. Furthermore, two studies have explored the effect of FD (and processing) on milk contaminated with common milk contaminants. Blackshaw et al showed that FD initially reduced bacterial counts of Staphylococcus Aureus (S. Aureus), Escherichia Coli (E. Coli) and Salmonella typhimurium (S. typhimirium) however without either gamma irradiation or HoP there was bacteria growth [43]. Jarynzyska et al. performed a similar study however; human milk was contaminated with five bacteria (E. Coli, S. Aureus, Listeria monocytogenes, cronobacter sakazakii and bacillus cereus). This study suggested that FD reduced E. Coli and Listeria monocytogenes and bacillus cereus growth (with Bacillus Cereus removed by 6 months storage). However, there was no effect on S. Aureus or Cronobacter sakazakii. High-pressure processing then FD lead to complete irradication of bacteria [32].
Human milk contains molecules that inhibit bacterial growth. No study has found that FD affects the bacterial growth inhibition or bactericidal capacity of human milk [36, 42, 43].
Clinical
Clinical studies using FD human milk have focused on providing this to preterm infants to provide the additional protein and calories they require for growth. These studies have had small sample sizes with a maximum of 42 infants reported in one study [44] (Table 5). The studies have mainly been cohort or observational designs, frequently focusing on the metabolic effects of a diet containing FD human milk. Four randomised controlled trials have been reported with a maximum of 40 infants per trial. Boehm et al compared the use of CMF to FD HM (as source of HM fortifier) showing no difference in growth, nitrogen or fat balance [45]. The primary focus of Bechensteen et al was the effect of erythropoietin on erythropoesis with the infants also randomised to receive FD HM or CMF as the source of HM fortifier [46]. Bechensteen et al found no effect on growth or erythropoeisis comparing these different diets [46]. Thomaz et al compared three diets in 24 infants with a primary outcome of phenylalanine levels showing that using CMF lead to significant increase in phenylalanine levels in comparison to using FD HM (as the source of HM fortifier) [23]. Nogueira et al reported a phase 1 double blind randomised controlled trial comparing the source of fortification (CMF versus FD HM) in 40 infants with a combined primary outcome of feed tolerance and gastro-intestinal complications (necrotising enterocolitis, sepsis, gastro-intestinal bleeding or perforation) reporting no difference in the primary outcome between groups [47].
There have been very few adverse events reported. Serum electrolyte disturbances have been the only adverse events reported namely; transient tyrosinaemia [48], hypocalcaemia, and hypophosphoraemia [49, 50]. Increased increased amino acid excretion and serum bile acids have also been reported but only in small for gestational age infants compared to appropriately grown infants [51].
Infants fed FD fortified human milk had similar weight gain when compared to cow’s milk formula fed infants [46, 49, 50], or in comparison to intra-uterine growth rates [52].
Studies have reported weight gains between 16 g/kg/day and 21.3 g/kg/day in infants fed human milk fortified with FD human milk [45, 49, 53,54,55,56,57]. An outlier was a study reporting offering human milk lyophilizate that was re-dissolved to preterm infants, they found the infants had a weight gain of only 7.6 g/kg/day, which was likely due to not re-dissolving the FD human milk in human milk [58].
Discussion
Summary of evidence
Our scoping review revealed that FD human milk results in a decrease in fat globule size, decreased growth of pathogenic bacteria, decreased bile salt-stimulated esterase and lipase, decreased vitamin C, decreased IgG and decreased IgM. When newborn infants’ have been fed FD human milk serum electrolyte disturbances have been reported, namely tyrosinaemia, low phenylalanine, hypophosphataemia, and hypocalcaemia although no adverse clinical outcomes have been reported when compared with diets using CMF. Weight gain appears similar in comparison to when CMF is used (Fig. 2).
Macro-nutrients seem relatively unaffected by FD. The milk fat globule size is reduced which may be advantageous in improving its bioavailability. Similar to fortification with bovine based HMF, meeting the macronutrient needs of preterm infant’s may be challenging when using FD HM fortified human milk [25]. This work by Fusch et al suggested that no lyophilization factor can be used to meet the macronutrient needs of preterm infants (without deficiency or excess in at least one macronutrient), it is plausible that the sane is true of the concentration of micronutrients [25]. Target fortification using human milk analysis may overcome this challenge and offers the possibility of providing an exclusive human milk diet or even an exclusive mothers’ own milk diet to preterm infants. Alternatively, the addition of a fat and lactose removal step in the processing of FD HM may positively influence these challenges [22]. There have been concerns regarding the osmolarity of feeds since a trial conducted in 1975 showed an association between very high osmolarity feeds (639 mOsm/l in this trial) and necrotising enterocolitis [59]. This led to the American Academy of Pediatrics (AAP) suggesting the osmolarity of milk feeds should not exceed 400 mOsm/l (approximate osmolality of 450 mOsm/kgH20) [60]. More recent publications by the AAP and ESPGHAN do not recommend an upper limit of osmolality or osmolarity for fortified milk, with ESPGHAN suggesting that there is not consistent evidence that feed osmolality between 300-500mOm/kg is unsafe [61, 62]. The reported osmolality of FD fortified HM of between 389 and 456 mOSm/kg is within these limits [12, 19].
The micronutrient composition of human milk is relatively unaffected with no reported increase in toxic elements. However, FD fortified HM has reduced calcium, phosphorous and vitamin C than CMF fortified HM which would need to be considered in the management of preterm infants.
The bioactive components of HM likely confer the health benefits of a human milk diet. A significant reduction in IgA, bile-stimulated lipase and enterase have been reported following FD. FD likely affects the immune cells (lymphocytes and stem cells) of HM however, this has not been investigated. These reductions are especially important if FD HM is used as a lyophilizate as it is unlikely to confer the same benefits as fresh MOM. However, if FD HM is used as a fortifier to MOM then the reduction is likely not as significant. A consideration is the effect of different FD conditions on all human milk components. Castro-Albarran et al. did explore different heating plate temperatures showing that the temperature effects the rate of sublimation as well as the immunoglobulin concentration in human milk [35].
FD appears to have a positive effect on reducing bacterial growth without affecting the natural bactericidal capacity of HM. While pasteurization of FD milk or using pasteurized DHM to produce FD fortifiers remains an option, current evidence support the safety of FD milk. Unpasteurized FD mother’s own milk may promote healthy colonization of the neonatal gut microbiome, as unpasteurized MOM compared to pasteurized DHM has been shown to significantly affect gut microbial composition whilst pasteurized human milk derived human milk fortifier have little impact compared to bovine-derived fortifiers [63].
The clinical effect of using FD HM as a HM fortifier appear promising with acceptable weight gain and no significant adverse events reported. Electrolyte disturbances (particularly hypocalcaemia and hypophosphoraemia) are common in preterm infants however the reported disturbances require further exploration especially considering the work by Grance et al demonstrating that the calcium and phosphorous content of FD human milk (combined with DHM) does not meet the ESPGHAN recommendations for preterm infants [19]. However, there is limited clinical evidence available and reported studies may not be representative of preterm infants at all gestations. Additionally, the available studies are limited by the reporting of the FD method used which makes reproducibility challenging.
Whilst the aim of this review was to identify the effect of FD on HM, we have also described the effect of storage on FD HM. It has been shown using sorption isotherm that FD HM is easily rehydrated, however this means that storage should be in humidity of less than 20% or a moisture protective package [35].
Limitations
While our review summarizes the current knowledge and potential use of FD human milk in preterm infants, there are several limitations to acknowledge. We did not have access to translation services meaning that articles not in English were excluded. Furthermore, effects of FD process (temperatures, pressure, milk state (frozen, raw) prior to FD and length of process) were not available in most reported studies. 10 different lyophilization machines were mentioned in the articles reviewed making it difficult to ascertain similarities between the FD methods.. Defining and standardizing optimal conditions for FD are required for use in clinical practice. We acknowledge that FD human milk often does not happen in isolation as many studies use DHM that has undergone HoP or other processing before FD. Tables 1–5 gives detail of the comparisons used in the studies described and whether there have been other processes explored in the reported analysis.
Conclusion
Freeze-drying human milk has effects on the nutrient, microbial and bioactive components of human milk. Freeze-dried human milk appears a possible way to improve storage and potentially offer preterm infants’ additional nutrients without exposure to cows’ milk. Further clinical studies are required to prove the efficacy and safety of FD HM in preterm infants given the limited data and considering the potential benefit of delivering an exclusive human milk diet to preterm infants as highlighted by international pediatric societies [61, 64].
References
Varshney D, Singh M. Lyophilized biologics and vaccines—modality-based approches. Varshney D, Singh M, editors: Springer; 2015. 401 p.
Adams GD, Cook I, Ward KR. The principles of freeze-drying. Cryopreservation and freeze-drying protocols. 2015:1257:121–43.
Johnson S, Evans TA, Draper ES, Field DJ, Manktelow BN, Marlow N, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. 2015;100:F301–8.
Eidelman AI. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed Med. 2012;7:323–4.
Spiegler J, Preuss M, Gebauer C, Bendiks M, Herting E, Gopel W, et al. Does breastmilk influence the development of bronchopulmonary dysplasia? J Pediatr. 2016;169:76–80 e4.
Gates A, Marin T, Leo G, Stansfield BK. Review of preterm human-milk nutrient composition. Nutr Clin Pract. 2021;36:1163–72.
Barresi AA, Pisano R, Fissore D, Rasetto V, Velardi SA, Vallan A, et al. Monitoring of the primary drying of a lyophilization process in vials. Chem Eng Process: Process Intensif. 2009;48:408–23.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
Cavazos-Garduño A, Serrano-Niño JC, Solís-Pacheco JR, Gutierrez-Padilla JA, González-Reynoso O, Garcia HS, et al. Effect of pasteurization, freeze-drying and spray drying on the fat globule and lipid profile of human milk. J Food Nutr Res. 2016;4:296–302.
Manin L, Rydlewski A, Galuch M, Pizzo J, Zappielo C, Senes C, et al. Evaluation of the lipid quality of lyophilized pasteurized human milk for six months by GC-FID and ESI-MS. J Braz Chem Soc. 2019;30:1579–86.
Bomfim VS, Jordao AA, Alves LG, Martinez FE, Camelo JS. Human milk enriched with human milk lyophilisate for feeding very low birth weight preterm infants: a preclinical experimental study focusing on fatty acid profile. Plos One. 2018;13:e0202794.
Oliveira MM, Aragon DC, Bomfim VS, Trevilato TMB, Alves LG, Heck AR, et al. Development of a human milk concentrate with human milk lyophilizate for feeding very low birth weight preterm infants: A preclinical experimental study. PLoS One. 2019;14:e0210999.
Lucas A, Lucas PJ, Chavin SI, Lyster RLJ, Baum JD. A human milk formula. Early Hum Dev. 1980;4:15–21.
Neia V, Santos P, Tavares C, Paula M, Costa S, Zacarias J, et al. Lipid Profile of Human Milk in Different Lactation Stages Submitted to Pasteurization, Lyophilization and Spray-Drying Processes. J Braz Chem Soc. 2023;34:54–62.
Friend BA, Shahani KM, Long CA, Agel EN. Evaluation of freeze-drying, pasteurization, high-temperature heating and storage on selected enzymes, B-vitamins and lipids of mature human milk. J Food Prot. 1983;46:330–4.
Blackshaw K, Wu J, Proschogo N, Davies J, Oldfield D, Schindeler A, et al. The effect of thermal pasteurization, freeze-drying, and gamma irradiation on donor human milk. Food Chem. 2022;373:131402.
Dill CW, Chen CT, Alford ES, Edwards RL, Richter RL, Garza C. Lipolytic activity during storage of human milk: accumulation of free fatty acids. J Food Prot. 1984;47:690–3.
Cortez MV, Soria EA. The effect of freeze-drying on the nutrient, polyphenol, and oxidant levels of breast milk. Breastfeed Med. 2016;11:551–4.
Grance TR, de Oliveira Serafin O, Thomaz DM, Palhares DB. Homologous fortifier for very low birth weight preterm infant feed. Rev Paul Pediatr. 2015;33:28–33.
Dhar J, Davidson AGF, Martinez FE, Barr S, Desai ID, Nakai S. Ultrasonication, lyophilization, freezing and storage effects on fat loss during mechanical infusion of expressed human milk. J Food Sci. 1995;60:375–7.
Hahn WH, Bae SP, Song S, Park S, Lee J, Seo JB, et al. The freeze-drying does not influence the proteomic profiles of human milk. J Matern Fetal Neonatal Med. 2020;33:2069–74.
Thomaz DM, Serafim PO, Palhares DB, Melnikov P, Venhofen L, Vargas MO. Comparison between homologous human milk supplements and a commercial supplement for very low birth weight infants. J Pediatr (Rio J). 2012;88:119–24.
Thomaz DM, Serafin PO, Palhares DB, Tavares LV, Grance TR. Serum phenylalanine in preterm newborns fed different diets of human milk. J Pediatr (Rio J). 2014;90:518–22.
Hahn WH, Kim J, Song S, Park S, Kang NM. The human milk oligosaccharides are not affected by pasteurization and freeze-drying. J Matern Fetal Neonatal Med. 2019;32:985–91.
Fusch S, Fusch G, Yousuf EI, Rochow M, So HY, Fusch C, et al. Individualized target fortification of breast milk: optimizing macronutrient content using different fortifiers and approaches. Front Nutr. 2021;8:652641.
Oliveira MM, Trevilato TMB, Segura-Munoz SI, Aragon DC, Alves LG, Nadal M, et al. Essential and toxic elements in human milk concentrate with human milk lyophilizate: a preclinical study. Environ Res. 2020;188:109733.
Barnett NW, Chen LS, Kirkbright GF. Determination of trace concentrations of lead and nickel in freeze-dried human milk by atomic absorption spectrometry and inductively-coupled plasma emission spectrometry. Anal Chim Acta. 1983;149:115–21.
Martysiak-Żurowska D, Puta M, Rodzik A, Malinowska-Pańczyk E. The effect of lyophilization on selected biologically active components (vitamin C, catalase, lysozyme), total antioxidant capacity and lipid oxidation in human milk. Zywnosc Nauka Technologia Jakosc/Food Sci Technol Qual. 2017;112:121–8.
Lozano B, Castellote AI, Montes R, Lopez-Sabater MC. Vitamins, fatty acids, and antioxidant capacity stability during storage of freeze-dried human milk. Int J Food Sci Nutr. 2014;65:703–7.
Sproat T, Payne RP, Embleton ND, Berrington J, Hambleton S. T cells in preterm infants and the influence of milk diet. Front Immunol. 2020;11:1035.
Carr LE, Virmani MD, Rosa F, Munblit D, Matazel KS, Elolimy AA, et al. Role of human milk bioactives on infants’ gut and immune health. Front Immunol. 2021;12:604080.
Jarzynka S, Strom K, Barbarska O, Pawlikowska E, Minkiewicz-Zochniak A, Rosiak E, et al. Combination of high-pressure processing and freeze-drying as the most effective techniques in maintaining biological values and microbiological safety of donor milk. Int J Environ Res Public Health. 2021;18:2147.
Dill CW, Chen CT, Alford ES, Edwards RL, Richter RL, Garza C. Lipolytic activity during storage of human milk: stability of the bile salt-stimulated lipase. J Food Prot. 1983;46:994–6.
O’Connor CJ, Longbottom JR, Walde P. Inactivation of bile-salt stimulated human milk esterase: effect of storage and heat. J Pediatr Gastroenterol Nutr. 1986;5:630–7.
Castro-Albarrán J, Aguilar-Uscanga BR, Calon F, St-Amour I, Solís-Pacheco J, Saucier L, et al. Spray and freeze drying of human milk on the retention of immunoglobulins (IgA, IgG, IgM). Dry Technol. 2016;34:1801–9.
Carbonare SB, Palmeira P, Silva MLM, Carneiro-Sampaio M. Effect of microwave radiation, pasteurization and lyophilization on the ability of human milk to inhibit Escherichia coli adherence to HEp-2 cells. J DIARRHOEAL Dis Res. 1996;14:90–4.
Hahn WH, Bae SP, Lee H, Park J, Park S, Kang NM. The impact of freeze-drying on the glycoproteomic profiles of human milk. Anal Sci Technol. 2020;33:177–85.
Martysiak-Żurowska D, Rozek P, Puta M. The effect of freeze-drying and storage on lysosyme activity, lactoferrin content, superoxide dismutase activity, total antioxidant capacity and fatty acid profile of freeze-dried human milk. Dry Technol. 2022;40:615–25.
Gila-Diaz A, Arribas SM, Algara A, Martin-Cabrejas MA, Lopez de Pablo AL, Saenz de Pipaon M, et al. A review of bioactive factors in human breastmilk: a focus on prematurity. Nutrients. 2019;11:1307.
Neia VJC, Zacarias JMV, Alencar JBD, Santos PDSD, Tavares CBG, Paula MG, et al. Effect of lyophilization and spray-drying on cytokine levels and antioxidant capacity in human milk. Dry Technol. 2021;40:3149–59.
Agel EN, Friend BA, Long CA, Shahani KM. Bacterial content of raw and processed human milk (1). J Food Prot. 1982;45:533–6.
Salcedo J, Gormaz M, Lopez-Mendoza MC, Nogarotto E, Silvestre D. Human milk bactericidal properties: effect of lyophilization and relation to maternal factors and milk components. J Pediatr Gastroenterol Nutr. 2015;60:527–32.
Blackshaw K, Wu J, Valtchev P, Lau E, Banati RB, Dehghani F, et al. The Effects of Thermal Pasteurisation, Freeze-Drying, and Gamma-Irradiation on the Antibacterial Properties of Donor Human Milk. Foods. 2021;10:2077.
Boehm G, Senger H, Muller D, Beyreiss K, Raiha NC. Metabolic differences between AGA- and SGA-infants of very low birthweight. II. Relationship to protein intake. Acta Paediatr Scand. 1988;77:642–6.
Boehm G, Raiha NC. Postmenstrual age correlates to indices of protein metabolism in very low birth weight infants. J Pediatr Gastroenterol Nutr. 1993;16:306–10.
Bechensteen AG, Haga P, Halvorsen S, Liestol K, Lindemann R, Whitelaw A, et al. Effect of low and moderate doses of recombinant human erythropoietin on the haematological response in premature infants on a high protein and iron intake. Eur J Pediatr. 1997;156:56–61.
Nogueira-Pileggi V, Achcar MC, Carmona F, Carnevale da Silva A, Aragon DC, da Veiga Ued F, et al. LioNeo project: a randomised double-blind clinical trial for nutrition of very-low-birth-weight infants. Br J Nutr. 2022;128:2490–7.
Hagelberg S, Lindblad BS, Lundsjo A, Carlsson B, Fonden R, Fujita H, et al. The protein tolerance of very low birth weight infants fed human milk protein enriched mother’s milk. Acta Paediatr Scand. 1982;71:597–601.
Schanler RJ, Garza C. Plasma amino acid differences in very low birth weight infants fed either human milk or whey-dominant cow milk formula. Pediatr Res. 1987;21:301–5.
Schanler RJ, Garza C, O’Brian Smith E. Fortified mothers’ milk for very low birth weight infants: Results of macromineral balance studies. Fetal Neonatal Med. 1985;107:767–74.
Boehm G, Melichar V, Lorenz I, Muller D, Beyreiss K. Nutrition of newborns small for gestational age with human milk lyophilisate enriched human milk during the first week of life. Acta Paediatr Hungarica. 1985;26:261–9.
Voyer M, Senterre J, Rigo J, Charlas J, Satge P. Human milk lacto-engineering. Growth nitrogen metabolism, and energy balance in preterm infants. Acta Paediatr Scand. 1984;73:302–6.
Boehm G, Senger H, Muller D, Beyreiss K, Raiha NC. Metabolic differences between AGA-and SGA-infants of very low birthweight. III. Influence of postnatal age. Acta Paediatr Scand. 1989;78:677–81.
Boehm G, Gedlu E, Muller DM, Beyreiss K, Raiha NCR. Relationship between urea and ammonium excretion in the urine of very-low-birth-weight infants appropriate for gestational age. Biomed Biochim Acta. 1990;49:69–74.
Boehm G, Geolu E, Muller MD, Beyreiss K, Raiha NCR. Postnatal development of urea and ammonia excretion in urine of very low birthweight infants small for gestational age. Acta Paediatr Hungarica. 1991;31:31–45.
Boehm G, Muller DM, Senger H, Borte M, Moro G. Nitrogen and fat balances in very low birth weight infants fed human milk fortified with human milk or bovine milk protein. Eur J Pediatr. 1993;152:236–9.
Boehm G, Müller MD, Senger H, Melichar V. Influence of postnatal age on nitrogen metabolism in very low birth weight infants appropriate for gestational age. Acta Paediatr Hungarica. 1990;30:423–33.
Boehm G, Melichar V, Müller DM, Mikova M. The application of redissolved human milk lyophilisate for nutrition of very low birth weight infants. Acta Paediatr Hungarica. 1987;28:267.
Book LS, Herbst JJ, Atherton SO, Jung AL. Necrotizing enterocolitis in fed an elemental formula low-birth-weight infants. J Pediatr. 1975;87:602–5.
Barness LA, Mauer AM, Holliday MA, Anderson AS, Dallman PR, Forbes GB, et al. Commentary on breast-feeding and infant formulas, including proposed standards for formulas. Pediatrics. 1976;57:278–85.
Embleton ND, Moltu SJ, Lapillonne A, Van Den Akker CH, Carnielli V, Fusch C, et al. Enteral nutrition in preterm infants (2022): a position paper from the ESPGHAN committee on nutrition and invited experts. J Pediatr Gastroenterol Nutr. 2023;76:248–68.
Kleinman RE, Greer FR, Nutrition AAoPCo. Pediatric nutrition: American Academy of Pediatrics Elk Grove Village, IL; 2014.
Kumbhare SV, Jones WD, Fast S, Bonner C, Jong G, Van Domselaar G, et al. Source of human milk (mother or donor) is more important than fortifier type (human or bovine) in shaping the preterm infant microbiome. Cell Rep Med. 2022;3:100712.
Mizuno K, Shimizu T, Ida S, Ito S, Inokuchi M, Ohura T, et al. Policy statement of enteral nutrition for preterm and very low birthweight infants. Pediatr Int. 2020;62:124–7.
Castro Albarran J, Navarro Hernandez RE, Solis Pacheco JR, Salazar Quinones IC, Macias Lopez GG, Barrera de Leon JC. et al.[Impact of pasteurization/freeze-dryingon available immunoglobulin content of the mature human milk. Use in human milk banking of hospitals].Nutr Hosp.2017;34:899–906.
Schanler RJ, Goldblum RM, Garza C, Goldman AS. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr Res. 1986;20:711–5.
Acknowledgements
We are very grateful to Caitlin McClurg (University of Calgary Librarian) for performing the initial literature search.
Author information
Authors and Affiliations
Contributions
BA & TDRS conceived and designed the work. TDRS/AG/BA reviewed the abstracts and full texts to identify suitable articles. TDRS charted and collated the data and drafted the initial manuscript. AG/BA reviewed and appraised the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sproat, T.D.R., Ghosh, A. & Alshaikh, B.N. Lyophilized (freeze-dried) human milk for preterm infants: a scoping review. J Perinatol 44, 612–627 (2024). https://doi.org/10.1038/s41372-023-01861-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01861-8
- Springer Nature America, Inc.