Abstract
Objective
Regionalized care reduces neonatal morbidity and mortality. This study evaluated the association of patient characteristics with quantitative differences in neonatal transport networks.
Study design
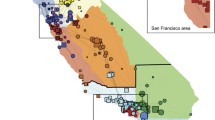
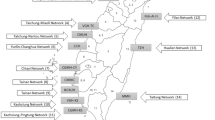
We retrospectively analyzed prospectively collected data for infants <28 days of age acutely transported within California from 2008 to 2012. We generated graphs representing bidirectional transfers between hospitals, stratified by patient attribute, and compared standard network analysis metrics.
Result
We analyzed 34,708 acute transfers, representing 1594 unique transfer routes between 271 hospitals. Density, centralization, efficiency, and modularity differed significantly among networks drawn based on different infant attributes. Compared to term infants and to those transported for medical reasons, network metrics identify greater degrees of regionalization for preterm and surgical patients (more centralized and less dense, respectively [p < 0.001]).
Conclusion
Neonatal interhospital transport networks differ by patient attributes as reflected by differences in network metrics, suggesting that regionalization should be considered in the context of a multidimensional system.


Similar content being viewed by others
References
Lupton BA, Pendray MR. Regionalized neonatal emergency transport. Semin Neonatol. 2004;9:125–33.
Yeast JD, Poskin M, Stockbauer JW, Shaffer S. Changing patterns in regionalization of perinatal care and the impact on neonatal mortality. Am J Obstet Gynecol. 1998;178:131–5.
Paneth N, Kiely JL, Wallenstein S, Marcus M, Pakter J, Susser M. Newborn intensive care and neonatal mortality in low-birth-weight infants: a population study. N Engl J Med. 1982;307:149–55.
Gortmaker S, Sobol A, Clark C, Walker DK, Geronimus A. The survival of very low-birth weight infants by level of hospital of birth: a population study of perinatal systems in four states. Am J Obstet Gynecol. 1985;152:517–24.
Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low-birth-weight and very preterm infants: a meta-analysis. JAMA. 2010;304:992–1000.
Rashidian A, Omidvari AH, Vali Y, Mortaz S, Yousefi-Nooraie R, Jafari M, et al. The effectiveness of regionalization of perinatal care services-a systematic review. Public Health. 2014;128:872–85.
Neto MT. Perinatal care in Portugal: effects of 15 years of a regionalized system. Acta Paediatr. 2006;95:1349–52.
Modanlou HD, Dorchester W, Freeman RK, Rommal C. Perinatal transport to a regional perinatal center in a metropolitan area—maternal versus neonatal transport. Am J Obstet Gynecol. 1980;138:1157–64.
Phibbs CS, Bronstein JM, Buxton E, Phibbs RH. The effects of patient volume and level of care at the hospital of birth on neonatal mortality. JAMA. 1996;276:1054–9.
Shlossman PA, Manley JS, Sciscione AC, Colmorgen GH. An analysis of neonatal morbidity and mortality in maternal (in utero) and neonatal transports at 24–34 weeks’ gestation. Am J Perinatol. 1997;14:449–56.
Cifuentes J, Bronstein J, Phibbs CS, Phibbs RH, Schmitt SK, Carlo WA. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109:745–51.
Lorch SA, Baiocchi M, Ahlberg CE, Small DS. The differential impact of delivery hospital on the outcomes of premature infants. Pediatrics. 2012;130:270–8.
Jensen EA, Lorch SA. Effects of a birth hospital’s neonatal intensive care unit level and annual volume of very low-birth-weight infant deliveries on morbidity and mortality. JAMA Pediatr. 2015;169:e151906.
Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N Engl J Med. 2007;356:2165–75.
Kaneko M, Yamashita R, Kai K, Yamada N, Sameshima H, Ikenoue T. Perinatal morbidity and mortality for extremely low-birthweight infants: a population-based study of regionalized maternal and neonatal transport. J Obstet Gynaecol Res. 2015;41:1056–66.
Mori R, Fujimura M, Shiraishi J, Evans B, Corkett M, Negishi H, et al. Duration of inter-facility neonatal transport and neonatal mortality: systematic review and cohort study. Pediatr Int. 2007;49:452–8.
Bowman E, Doyle LW, Murton LJ, Roy R, Kitchen WH. Increased mortality of preterm infants transferred between tertiary perinatal centres. BMJ. 1988;297:1098–100.
Palmer KG, Kronsberg SS, Barton BA, Hobbs CA, Hall RW, Anand KJ. Effect of inborn versus outborn delivery on clinical outcomes in ventilated preterm neonates: secondary results from the NEOPAIN trial. J Perinatol. 2005;25:270–5.
Mohamed MA, Aly H. Transport of premature infants is associated with increased risk for intraventricular haemorrhage. Arch Dis Child-Fetal Neonatal Ed. 2010;95:F403–7.
Arora P, Bajaj M, Natarajan G, Arora NP, Kalra VK, Zidan M, et al. Impact of interhospital transport on the physiologic status of very low-birth-weight infants. Am J Perinatol. 2014;31:237–44.
Helenius K, Longford N, Lehtonen L, Modi N, Gale C, Neonatal Data Analysis U, et al. Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ. 2019;367:l5678
Kunz SN, Zupancic JA, Rigdon J, Phibbs CS, Lee HC, Gould JB, et al. Network analysis: a novel method for mapping neonatal acute transport patterns in California. J Perinatol. 2017;37:702–8.
Das K, Samanta S, Pal M. Study on centrality measures in social networks: a survey. Soc Netw Anal Min. 2018;8:1–13.
Benzi M, Klymko C. On the limiting behavior of parameter-dependent network centrality measures. SIAM J Matrix Anal Appl. 2015;36:686–706.
Katz L. A new status index derived from sociometric analysis. Psychometrika. 1953;18:39–43.
de Laat M, Lally V, Lipponen L, Simons RJ. Investigating patterns of interaction in networked learning and computer-supported collaborative learning: a role for social network analysis. Int J Comp-Supp Coll. 2007;2:87–103.
Pasquaretta C, Leve M, Claidiere N, van de Waal E, Whiten A, MacIntosh AJJ, et al. Social networks in primates: smart and tolerant species have more efficient networks. Sci Rep. 2014;4:7600.
Latora V, Marchiori M. A measure of centrality based on network efficiency. New J Phys. 2007;9:188.
Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701.
Yang Z, Algesheimer R, Tessone CJ. A comparative analysis of community detection algorithms on artificial networks. Sci Rep. 2016;6:30750.
Fortunato S. Community detection in graphs. Phys Rep. 2010;486:75–174.
Newman MEJ. Modularity and community structure in networks. Proc Natl Acad Sci U S A. 2006;103:8577–82.
Pons P, Latapy M. Computing communities in large networks using random walks. Lecture Notes in Computer Science, vol. 3733. 2005. p. 284–93.
Profit J, Gould JB, Bennett M, Goldstein BA, Draper D, Phibbs CS, et al. The association of level of care with NICU quality. Pediatrics. 2016;137:e20144210.
Gould JB, Marks AR, Chavez G. Expansion of community-based perinatal care in California. J Perinatol. 2002;22:630–40.
Odén A, Wedel H. Arguments for Fisher’s permutation test. Ann Stat. 1975;3:518–20.
Fisher RA. Statistical methods for research workers. In: Breakthroughs in statistics. Springer; 1992. p. 66–70.
Simpson SL, Lyday RG, Hayasaka S, Marsh AP, Laurienti PJ. A permutation testing framework to compare groups of brain networks. Front Comput Neurosci. 2013;7:171.
Efron B. Nonparametric estimates of standard error—the jackknife, the bootstrap and other methods. Biometrika. 1981;68:589–99.
Hagberg A, Swart P, S Chult D. Exploring network structure, dynamics, and function using NetworkX. Los Alamos National Laboratory (LANL), Los Alamos, NM (United States); 2008.
Csardi G, Nepusz T. The igraph software package for complex network research. InterJournal, Complex Syst. 2006;1695:1–9.
Rives AW, Galitski T. Modular organization of cellular networks. Proc Natl Acad Sci U S A. 2003;100:1128–33.
Funding
SNK was supported by Grant K08 HS025749 from the Agency for Healthcare Research and Quality. JP and DH were supported by the Stanford Maternal Child Health Research Institute (MCHRI) Grant Program.
Author information
Authors and Affiliations
Contributions
SNK conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. JAFZ and JP conceptualized and designed the study, and reviewed and revised the manuscript. MZ, JR, and DH carried out the analyses, and reviewed and revised the manuscript. CSP conceptualized and designed the study, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Supplementary information is available at JPER’s website.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was approved by the institutional review board at Stanford University (protocol #50047). The study was submitted to the institutional review board at Beth Israel Deaconess Medical Center and deemed not human subject research, as all analysis was performed at Stanford University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kunz, S.N., Helkey, D., Zitnik, M. et al. Quantifying the variation in neonatal transport referral patterns using network analysis. J Perinatol 41, 2795–2803 (2021). https://doi.org/10.1038/s41372-021-01091-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01091-w
- Springer Nature America, Inc.
This article is cited by
-
Maternal and neonatal risk-appropriate care: gaps, strategies, and areas for further research
Journal of Perinatology (2023)