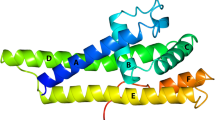
Abstract
Borrelia burgdorferi, a spirochete transmitted to human hosts during feeding of infected Ixodes ticks, is the causative agent of Lyme disease. Serum-resistant B. burgdorferi strains cause a chronic, multisystemic form of the disease and bind complement factor H (FH) and FH-like protein 1 (FHL-1) on the spirochete surface. Here we report the atomic structure for the key FHL-1- and FH-binding protein BbCRASP-1 and reveal a homodimer that presents a novel target for drug design.


Similar content being viewed by others
References
Barbour, A.G. & Hayes, S.F. Microbiol. Rev. 50, 381–400 (1986).
Steere, A.C. N. Engl. J. Med. 321, 586–596 (1989).
Wang, G.Q., van Dam, A.P., Schwartz, I. & Dankert, J. Clin. Microbiol. Rev. 12, 633–653 (1999).
Centers for Disease Control and Prevention (CDC). MMWR Morb. Mortal. Wkly Rep. 51, 29–31 (2002).
O'Connell, S., Granstrom, M., Gray, J.S. & Stanek, G. Zentralbl. Bakteriol. 287, 229–240 (1998).
Stanek, G. & Strle, F. Lancet 362, 1639–1647 (2003).
Kraiczy, P. et al. J. Biol. Chem. 279, 2421–2429 (2004).
Kraiczy, P., Skerka, C., Brade, V. & Zipfel, P.F. Infect. Immun. 69, 7800–7809 (2001).
Kraiczy, P., Skerka, C., Kirschfink, M., Brade, V. & Zipfel, P.F. Eur. J. Immunol. 31, 1674–1684 (2001).
Kraiczy, P., Skerka, C., Kirschfink, M., Zipfel, P.F. & Brade, V. Int. Immunopharmacol. 1, 393–401 (2001).
Lea, S.M. et al. J. Biol. Chem. 273, 30443–30447 (1998).
Cordes, F.S. et al. Acta Crystallogr. D 60, 929–932 (2004).
Holm, L. & Sander, C. J. Mol. Biol. 233, 123–138 (1993).
Lesk, A. Introduction to Protein Science: Architecture, Function, and Genomics (Oxford Univ. Press, Oxford, 2004).
Acknowledgements
Thanks to M. Walsh at BM14 and the staff of ID14-4 and ID29 at the European Synchrotron Radiation Facility for assistance with data collection and to M. Morgan for assistance with the crystallization robot in the Laboratory of Molecular Biophysics, Oxford. The authors are indebted for the financial support of the Wellcome Trust (studentship to F.S.C., no. 059380), the UK Biotechnology and Biological Sciences Research Council (43/B16601 to S.M.L.), the UK Medical Research Council and the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Data collection, phasing and refinement statistics. (PDF 63 kb)
Rights and permissions
About this article
Cite this article
Cordes, F., Roversi, P., Kraiczy, P. et al. A novel fold for the factor H–binding protein BbCRASP-1 of Borrelia burgdorferi. Nat Struct Mol Biol 12, 276–277 (2005). https://doi.org/10.1038/nsmb902
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb902
- Springer Nature America, Inc.
This article is cited by
-
High conservation combined with high plasticity: genomics and evolution of Borrelia bavariensis
BMC Genomics (2020)
-
Crystal structure of the membrane attack complex assembly inhibitor BGA71 from the Lyme disease agent Borrelia bavariensis
Scientific Reports (2018)
-
Binding of human complement regulators FHL-1 and factor H to CRASP-1 orthologs of Borrelia burgdorferi
Wiener klinische Wochenschrift (2006)
-
Evolving models of Lyme disease spirochete gene regulation
Wiener klinische Wochenschrift (2006)