Abstract
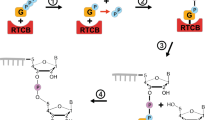
The hammerhead ribozyme is a more efficient ribonuclease than an RNA ligase. Under typical reaction conditions, the rate of RNA chain cleavage is ∼100-fold faster than the rate of the reverse ligation reaction such that virtually all of the hammerhead is in its cleaved form at equilibrium. Here we show that the introduction of a crosslink away from the catalytic core of the hammerhead has little effect on the cleavage rate but dramatically increases the ligation rate, thereby making the hammerhead an efficient RNA ligase. This experiment emphasizes the role of molecular flexibility in defining the properties of a macromolecular catalyst and suggests why other small ribozymes are more efficient ligases than ribonucleases.



Similar content being viewed by others
Accession codes
References
Hutchins, C.J., Rathjen, P.D., Forster, A.C. & Symons, R.H. Nucleic Acids Res. 14, 3627–3640 (1986).
Hertel, K.J., Herschlag, D. & Uhlenbeck, O.C. Biochemistry 33, 3374–3385 (1994).
Hertel, K.J. & Uhlenbeck, O.C. Biochemistry 34, 1744–1749 (1995).
Scott, W.G., Finch, J.T. & Klug, A. Cell 81, 991–1002 (1995).
Stage-Zimmermann, T.K. & Uhlenbeck, O.C. Biochemistry 37, 9386–9393 (1998).
Sigurdsson, S.T., Tuschl, T. & Eckstein, F. RNA 1, 575–583 (1995).
Cohen, S.B. & Cech, T.R. J. Am. Chem. Soc. 119, 6259–6268 (1997).
Fedor, M.J. & Uhlenbeck, O.C. Proc. Natl. Acad. Sci. USA 87, 1668–1672 (1990).
Fedor, M.J. & Uhlenbeck, O.C. Biochemistry 31, 12042–12054 (1992).
Slim, G. & Gait, M.J. Nucleic Acids Res. 19, 1183–1188 (1991).
Perreault, J.P., Labuda, D., Usman, N., Yang, J.-H. & Cedergren, R. Biochemistry 30, 4020–4025 (1991).
Perkins, T.A., Wolf, D.E. & Goodchild, J. Biochemistry 35, 16370–16377 (1996).
Clouet-d'Orval, B. & Uhlenbeck, O.C. RNA 2, 483–491 (1996).
Long, D.M., LaRiviere, F.J. & Uhlenbeck, O.C. Biochemistry 34, 14435–14440 (1995).
Murray, J.B., Szoke, H., Szoke, A. & Scott, W.G. Mol. Cell 5, 279–287 (2000).
Simorre, J.P., Legault, P., Hangar, A.B., Michiels, P. & Pardi, A. Biochemistry 36, 518–525 (1997).
Bassi, G.S., Murchie, A.I.H. & Lilley, D.M. RNA 2, 756–768 (1996).
Amiri, K.M.A. & Hagerman, P.J. J. Mol. Biol. 261, 125–134 (1996).
Cole, P.E. & Crothers, D.M. Biochemistry 11, 4368–4374 (1972).
Zarrinkar, P.P. & Williamson, J.R. Nature Struct. Biol. 3, 432–438 (1996).
Fang, X.W., Pan, T. & Sosnick, T.R. Nature Struct. Biol. 6, 1091–1095 (1999).
Hegg, L.A. & Fedor, M.J. Biochemistry 34, 15813–15828 (1995).
Berzal-Herranz, A., Joseph, S. & Burke, J.M. Genes Dev. 6, 129–134 (1992).
Saville, B.J. & Collins, R.A. Proc. Natl. Acad. Sci. USA 88, 8826–8830 (1991).
Beattie, T.L. & Collins, R.A. J. Mol. Biol. 267, 830–840 (1997).
Esteban, J.A., Banerjee, A.R. & Burke, J.M. J. Biol. Chem. 272, 13629–13639 (1997).
Fedor, M.J. Biochemistry 38, 11040–11050 (1999).
Rupert, P.B. & Ferre-D'Amare, A.R. Nature 410, 780–786 (2001).
Beaudry, A., DeFoe, J., Zinnen, S., Burgin, A. & Beigelman, L. Chem. Biol. 7, 323–334 (2000).
Santoro, S.W. & Joyce, G.F. Proc. Natl. Acad. Sci. USA 94, 4262–4266 (1997).
Pan, T. & Uhlenbeck, O.C. Nature 358, 560–563 (1992).
Chervitz, S.A. & Falke, J.J. J. Biol. Chem. 270, 24043–24053 (1995).
Hertel, K.J. et al. Nucleic Acids Res. 20, 3252 (1992).
Acknowledgements
Special thanks to S. Cohen for assistance with the thiol–disulfide interchange crosslinking and W. Pieken for supplying the 2′ amino phosphoramidites used in this study. This research was supported by a grant from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stage-Zimmermann, T., Uhlenbeck, O. A covalent crosslink converts the hammerhead ribozyme from a ribonuclease to an RNA ligase. Nat Struct Mol Biol 8, 863–867 (2001). https://doi.org/10.1038/nsb1001-863
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1001-863
- Springer Nature America, Inc.