Key Points
-
The three Rnd proteins appeared late in evolution (in vertebrates) and are Rho-family proteins with unusual biochemical properties. They do not cycle between GDP (inactive) and GTP (active) states, but are constitutively active (GTP bound).
-
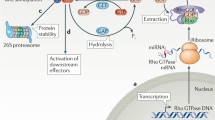
Because Rnd proteins are constitutively active and do not cycle, they are regulated by other mechanisms, including expression levels, localization, phosphorylation and degradation.
-
Rnd proteins have effects antagonistic to Rho, and they inhibit the formation of contractile acto-myosin cables (stress fibres). Rnd proteins are expressed in neurons where they participate in the extension of growth cones.
-
Rnd proteins are involved in axon guidance and interact with plexins (the semaphorin receptors) to modulate downstream pathways. Rnd proteins are also involved in fibroblast-growth-factor-receptor-1 signalling.
-
Rnd3 overexpression blocks cell-cycle progression, and Rnd3 protein expression is frequently decreased in prostate and breast cancer. However, increased expression of Rnd3 has also been observed in cancers with a constitutively active Ras–Raf pathway. Additional studies are required to understand the basis for these apparent discrepancies.
-
Further studies in animal models are needed to investigate the roles of Rnd proteins in brain development, and a more precise understanding of the functions of Rnd partners is required. A role in axonal regeneration of injured nerve cells is another exciting possibility.
Abstract
The Rnd proteins, which form a distinct sub-group of the Rho family of small GTP-binding proteins, have been shown to regulate the organization of the actin cytoskeleton in several tissues. In the brain, they participate in neurite extension, whereas in smooth muscle, they modulate contractility. Recent evidence has shown that Rnd3 (RhoE) is also involved in the regulation of cell-cycle progression and transformation, indicating that these proteins might have other, as yet unexplored roles.




Similar content being viewed by others
References
Wherlock, M. & Mellor, H. The Rho GTPase family: a Racs to Wrchs story. J. Cell Sci. 115, 239–240 (2002).
Philips, A. et al. Ascidians as a vertebrate-like model organism for physiological studies of Rho GTPase signaling. Biol. Cell 95, 295–302 (2003).
Wunnenberg-Stapleton, K., Blitz, I. L., Hashimoto, C. & Cho, K. W. Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development 126, 5339–5351 (1999).
Foster, R. et al. Identification of a novel human Rho protein with unusual properties: GTPase deficiency and in vivo farnesylation. Mol. Cell Biol. 16, 2689–2699 (1996). First description of Rnd3/RhoE and some of its biological properties.
Nobes, C. D. et al. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J. Cell Biol. 141, 187–197 (1998). First description of the complete Rnd protein family, its biochemical properties, localization and the effects of heterologous expression on the actin cytoskeleton of fibroblasts and MDCK cells.
Garavini, H. et al. Crystal structure of the core domain of Rnd3/RhoE: a constitutively activated small G protein. Biochemistry 41, 6303–6310 (2002).
Fiegen, D., Blumenstein, L., Stege, P., Vetter, I. R. & Ahmadian, M. R. Crystal structure of Rnd3/RhoE: functional implications. FEBS Lett. 525, 100–104 (2002).
Riento, K., Guasch, R. M., Garg, R., Jin, B. & Ridley, A. J. RhoE binds to ROCK I and inhibits downstream signaling. Mol. Cell Biol. 23, 4219–4229 (2003). One of the mechanisms explaining the effects of Rnd3 on the actin cytoskeleton.
Guasch, R. M., Scambler, P., Jones, G. E. & Ridley, A. J. RhoE regulates actin cytoskeleton organization and cell migration. Mol. Cell Biol. 18, 4761–4771 (1998).
Aoki, J., Katoh, H., Mori, K. & Negishi, M. Rnd1, a novel rho family GTPase, induces the formation of neuritic processes in PC12 cells. Biochem. Biophys. Res. Commun. 278, 604–608 (2000).
Ishikawa, Y., Katoh, H. & Negishi, M. A role of Rnd1 GTPase in dendritic spine formation in hippocampal neurons. J. Neurosci. 23, 11065–11072 (2003).
Wennerberg, K. et al. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr. Biol. 13, 1106–1115 (2003). Probably the most important target to explain Rnd1 and Rnd3 effects on the actin cytoskeleton.
Vayssiere, B. et al. Interaction of the Grb7 adapter protein with Rnd1, a new member of the Rho family. FEBS Lett. 467, 91–96 (2000).
Han, D. C., Shen, T. L., Miao, H., Wang, B. & Guan, J. L. EphB1 associates with Grb7 and regulates cell migration. J. Biol. Chem. 277, 45655–45661 (2002).
Nishi, M. et al. RhoN, a novel small GTP-binding protein expressed predominantly in neurons and hepatic stellate cells. Brain Res. Mol. Brain Res. 67, 74–81 (1999).
Fujita, H., Katoh, H., Ishikawa, Y., Mori, K. & Negishi, M. Rapostlin is a novel effector of Rnd2 GTPase inducing neurite branching. J. Biol. Chem. 277, 45428–45434 (2002).
Kakimoto, T., Katoh, H. & Negishi, M. Identification of splicing variants of Rapostlin, a novel Rnd2 effector that interacts with neural Wiskott-Aldrich syndrome protein and induces neurite branching. J. Biol. Chem. 279, 14104–14110 (2004).
Katoh, H., Harada, A., Mori, K. & Negishi, M. Socius is a novel Rnd GTPase-interacting protein involved in disassembly of actin stress fibers. Mol. Cell Biol. 22, 2952–2964 (2002).
Naud, N. et al. Rho family GTPase Rnd2 interacts and co-localizes with MgcRacGAP in male germ cells. Biochem. J. 372, 105–112 (2003). Description of a function for Rnd2 in the testis where it is expressed at the highest levels.
Yuce, O., Pieknym A., Glotzer, M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170, 571–582 (2005).
Kruger, R. P., Aurandt, J. & Guan, K. -L. Semaphorins command cells to move. Nature Rev. Mol. Cell Biol. 6, 789–800 (2005).
Oinuma, I., Ishikawa, Y., Katoh, H. & Negishi, M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 305, 862–865 (2004). A milestone paper describing how the activation of the receptor plexin-B1 and its interaction with Rnd1 stimulates R-RasGAP activity and contributes to growth-cone collapse, in conjunction with PDZ–RhoGEF activation of RhoA (see also reference 23).
Oinuma, I., Katoh, H., Harada, A. & Negishi, M. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells. J. Biol. Chem. 278, 25671–25677 (2003). First description, in a heterologous system, of how the activation of the receptor plexin-B1 and its interaction with Rnd1 stimulates PDZ–RhoGEF, indicating how this contributes to growth-cone collapse in neurons.
Rohm, B., Rahim, B., Kleiber, B., Hovatta, I. & Puschel, A. W. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett. 486, 68–72 (2000). First description of a Ras GAP activity in semaphorins, which is modulated by Rnd1 or Rac.
Zanata, S. M., Hovatta, I., Rohm, B. & Puschel, A. W. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J. Neurosci. 22, 471–477 (2002).
Barberis, D. et al. Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J. 18, 592–594 (2004).
Song, H. et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281, 1515–1518 (1998).
Sauzeau, V. et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 275, 21722–21729 (2000).
Harada, A., Katoh, H. & Negishi, M. Direct interaction of Rnd1 with FRS2β regulates Rnd1-induced down-regulation of RhoA activity and is involved in fibroblast growth factor-induced neurite outgrowth in PC12 cells. J. Biol. Chem. 280, 18418–18424 (2005). Indicates how FGFR1 activates Rnd1.
Billuart, P., Winter, C. G., Maresh, A., Zhao, X. & Luo, L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell 107, 195–207 (2001).
Brouns, M. R., Matheson, S. F. & Settleman, J. p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nature Cell Biol. 3, 361–367 (2001).
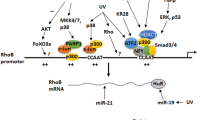
Hansen, S. H. et al. Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular signal-regulated kinase pathway. Mol. Cell Biol. 20, 9364–9375 (2000).
Murakami, T., Fujimoto, M., Ohtsuki, M. & Nakagawa, H. Expression profiling of cancer-related genes in human keratinocytes following non-lethal ultraviolet B irradiation. J. Dermatol. Sci. 27, 121–129 (2001).
Villalonga, P., Guasch, R. M., Riento, K. & Ridley, A. J. RhoE inhibits cell cycle progression and Ras-induced transformation. Mol. Cell Biol. 24, 7829–7840 (2004). First description of the effects of Rnd3 on the cell cycle and transformation.
Cario-Toumaniantz, C. et al. Modulation of RhoA-Rho kinase-mediated Ca2+ sensitization of rabbit myometrium during pregnancy — role of Rnd3. J. Physiol. 52, 403–413 (2003). First study showing that Rnd3 expression inhibits smooth-muscle contraction in a physiological situation.
Kim, Y. S., Kim, B., Karaki, H., Hori, M. & Ozaki, H. Up-regulation of Rnd1 during pregnancy serves as a negative-feedback control for Ca2+ sensitization of contractile elements in rat myometrium. Biochem. Biophys. Res. Commun. 311, 972–978 (2003).
Loirand, G., Cario-Toumaniantz, C., Chardin, P. & Pacaud, P. The Rho-related protein Rnd1 inhibits Ca2+ sensitization of rat smooth muscle. J. Physiol. 516, 825–834 (1999).
Cario-Toumaniantz, C. et al. Role of Rho kinase signalling in healthy and varicose human saphenous veins. Br. J. Pharmacol. 137, 205–212 (2002).
Camerer, E., Gjernes, E., Wiiger, M., Pringle, S. & Prydz, H. Binding of factor VIIa to tissue factor on keratinocytes induces gene expression. J. Biol. Chem. 275, 6580–6585 (2000).
Nishigaki, M. et al. Discovery of aberrant expression of R-RAS by cancer-linked DNA hypomethylation in gastric cancer using microarrays. Cancer Res. 65, 2115–2124 (2005).
Warton, K., Foster, N. C., Gold, W. A. & Stanley, K. K. A novel gene family induced by acute inflammation in endothelial cells. Gene 42, 85–95 (2004).
Jiang, H., Van De Ven, C., Satwani, P., Baxi, L. V. & Cairo, M. S. Differential gene expression patterns by oligonucleotide microarray of basal versus lipopolysaccharide-activated monocytes from cord blood versus adult peripheral blood. J. Immunol. 172, 5870–5879 (2004).
Chardin, P. GTPase regulation: getting aRnd Rock and Rho inhibition. Curr. Biol. 13, 702–704 (2003).
Riento, K. et al. RhoE function is regulated by ROCK I-mediated phosphorylation. EMBO J. 24, 1170–1180 (2005). Demonstration that Rock mediates Rnd3 phosphorylation and regulates its activity.
Riento, K. & Ridley, A. J. Rocks: multifunctional kinases in cell behaviour. Nature Rev. Mol. Cell Biol. 4, 446–456 (2003).
Doye, A. et al. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111, 553–564 (2002).
Wang, H. R. et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302, 1775–1779 (2003).
Krugmann, S., Williams, R., Stephens, L. & Hawkins, P. T. ARAP3 is a PI3K- and rap-regulated GAP for RhoA. Curr. Biol. 14, 1380–1384 (2004).
Rolli-Derkinderen, M. et al. Phosphorylation of serine 188 protects RhoA from ubiquitin/proteasome-mediated degradation in vascular smooth muscle cells. Circ. Res. 96, 1152–1160 (2005).
Coleman, M. L., Marshall, C. J. & Olson, M. F. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nature Rev. Mol. Cell Biol. 5, 355–366 (2004).
Bektic, J. et al. Small G-protein RhoE is underexpressed in prostate cancer and induces cell cycle arrest and apoptosis. Prostate 64, 332–340 (2005). Convincing set of data indicating that decreased Rnd3 expression can contribute to transformation.
Rubenstein, N. M., Chan, J. F., Kim, J. Y., Hansen, S. H. & Firestone, G. L. Rnd3/RhoE induces tight junction formation in mammary epithelial tumor cells. Exp. Cell Res. 305, 74–82 (2005).
Gress, T. M. et al. A pancreatic cancer-specific expression profile. Oncogene 13, 1819–1830 (1996).
Akashi, H., Han, H. J., Iizaka, M. & Nakamura, Y. Growth-suppressive effect of non-steroidal anti-inflammatory drugs on 11 colon-cancer cell lines and fluorescence differential display of genes whose expression is influenced by sulindac. Int. J. Cancer 88, 873–880 (2000).
Decourt, B., Bouleau, Y., Dulon, D. & Hafidi, A. Expression analysis of neuroleukin, calmodulin, cortactin, and Rho7/Rnd2 in the intact and injured mouse brain. Brain Res. Dev. Brain Res. 159, 36–54 (2005).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Huang, H. et al. Gene expression profiling and subgroup identification of oligodendrogliomas. Oncogene 23, 6012–6022 (2004).
Pomeroy, S. L. et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415, 436–442 (2002).
Trojan, L. et al. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 5, 183–191 (2005).
Bektic, J. et al. Identification of genes involved in estrogenic action in the human prostate using microarray analysis. Genomics 83, 34–44 (2004).
Liu, T. et al. Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Res. 63, 5034–5040 (2003); erratum in 64, 220 (2004).
Guo, S., Russo, I. H. & Russo, J. Difference in gene expression profile in breast epithelial cells from women with different reproductive history. Int. J. Oncol. 23, 933–941 (2003).
Jiang, W. G. et al. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin. Cancer Res. 9, 6432–6440 (2003).
van Groningen, J. J., Cornelissen, I. M., van Muijen, G. N., Blpeùers; H. P. & Swart, G. W. Simultaneous suppression of progression marker genes in the highly malignant human melanoma cell line BLM after transfection with the adenovirus-5 E1A gene. Biochem. Biophys. Res. Commun. 225, 808–816 (1996).
Smith T. M. et al. Complete genomic sequence and analysis of 117 kb of human DNA containing the gene BRCA1. Genome Res. 6, 1029–1049 (1996).
Acknowledgements
I would like to thank A. Ridley, A. Püschel, M. Negishi and P. Pacaud for sharing results before publication and for stimulating discussions, and I. Mus-Veteau for helpful comments. P.C. is supported by INSERM, Association pour la Recherche sur le Cancer, La Ligue Contre le Cancer, and Fondation pour la Recherche Médicale.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
UniProtKB
FURTHER INFORMATION
Glossary
- Guanine nucleotide-exchange factor
-
(GEF). A protein that facilitates the exchange of GDP (guanosine diphosphate) for GTP (guanosine triphosphate) in the nucleotide-binding pocket of a GTP-binding protein.
- GTPase-activating protein
-
(GAP). A protein that stimulates the intrinsic ability of a GTPase to hydrolyse GTP to GDP. GAPs negatively regulate GTPases by converting them from active (GTP-bound) to inactive (GDP-bound). There are approximately 70 different RhoGAPs in the human genome; p190 RhoGAP is one of the most highly and ubiquitously expressed.
- Guanine nucleotide-dissociation inhibitor
-
(GDI). A protein that interacts with a small GTP-binding protein and covers its lipid modification to form a cytosolic complex. It also inhibits the dissociation of GDP and therefore its replacement by GTP in a small GTP-binding protein, thereby maintaining it in an inactive cytosolic state.
- Geranylgeranyl
-
A 20 carbon (C20) prenyl modification that is added to the C terminus of Rho-family proteins by geranylgeranyl transferase.
- Ascidians
-
Chordates that are more closely related to vertebrates than flies or nematodes.
- Farnesylated
-
The addition of a 15 carbon (C15) lipid modification to the C terminus of Rho-family proteins by farnesyl transferase.
- Rock I and Rock II
-
Serine/threonine kinases that are Rho·GTP and control the phosphorylation of myosin light chain by phosphorylating and inhibiting myosin-light-chain phosphatase. Rocks are among the best-characterized Rho effectors.
- Lamellipodia
-
Thin, flat extensions at the cell periphery that are filled with a branching meshwork of actin filaments.
- Filopodia
-
Thin, transient actin protrusions that extend out from the cell surface and are formed by the elongation of bundled actin filaments in its core.
- PDZ–RhoGEF
-
A small protein domain that specifically binds the C-terminal motifs of some proteins (frequently membrane-associated), allowing the formation of a multi-protein complex.
- Growth-factor-receptor-bound-protein-2
-
(GRB2). An adaptor protein with one SH2 and two SH3 domains. The SH2 domain binds specific motifs with a phosphorylated tyrosine on several receptors or adaptors such as Shc, and the two SH3 domains bind proline-rich motifs of Sos (a Ras activator) and other signalling proteins.
- SH2-containing protein tyrosine phosphatase-2
-
(SHP2). Its two SH2 domains interact with many receptors, adhesion molecules, adaptors and other tyrosine-phosphorylated proteins that it might subsequently dephosphorylate owing to its phosphatase activity.
Rights and permissions
About this article
Cite this article
Chardin, P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol 7, 54–62 (2006). https://doi.org/10.1038/nrm1788
Issue Date:
DOI: https://doi.org/10.1038/nrm1788
- Springer Nature Limited
This article is cited by
-
Molecular basis and current insights of atypical Rho small GTPase in cancer
Molecular Biology Reports (2024)
-
Essential role of Rnd1 in innate immunity during viral and bacterial infections
Cell Death & Disease (2022)
-
Rnd3 is necessary for the correct oligodendrocyte differentiation and myelination in the central nervous system
Brain Structure and Function (2022)
-
RND2 attenuates apoptosis and autophagy in glioblastoma cells by targeting the p38 MAPK signalling pathway
Journal of Experimental & Clinical Cancer Research (2020)
-
Identification and characterization of a new isoform of small GTPase RhoE
Communications Biology (2020)