Abstract
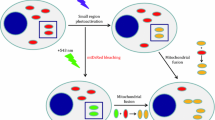
A general protocol is described to improve the specificity for imaging superoxide formation in live cells via fluorescence microscopy with either hydroethidine (HE) or its mitochondrially targeted derivative Mito-HE (MitoSOX Red). Two different excitation wavelengths are used to distinguish the superoxide-dependent hydroxylation of Mito-HE (385–405 nm) from the nonspecific formation of ethidium (480–520 nm). Furthermore, the dual wavelength imaging in live cells can be combined with immunocolocalization, which allows superoxide formation to be compared simultaneously in cocultures of two types of genetically manipulated cells in the same microscopic field. The combination of these approaches can greatly improve the specificity for imaging superoxide formation in cultured cells and tissues.



Similar content being viewed by others
References
Shigenaga, M.K., Hagen, T.M. & Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 91, 10771–10778 (1994).
Green, K., Brand, M.D. & Murphy, M.P. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53 (suppl 1): S110–118 (2004).
Rothe, G. & Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J. Leukoc. Biol. 47, 440–448 (1990).
Perticarari, S., Presani, G. & Banfi, E. A new flow cytometric assay for the evaluation of phagocytosis and the oxidative burst in whole blood. J. Immunol. Methods 170, 117–124 (1994).
Zhao, H. et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 34, 1359–1368 (2003).
Robinson, K.M. et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 103, 15038–15043 (2006).
Zielonka, J. et al. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic. Biol. Med. 44, 835–846 (2008).
Zhao, H. et al. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 102, 5727–5732 (2005).
Pehar, M. et al. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J. Neurosci. 27, 7777–7785 (2007).
Cassina, P. et al. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J. Neurosci. Res. 67, 21–29 (2002).
Zielonka, J., Vasquez-Vivar, J. & Kalyanaraman, B. The confounding effects of light, sonication, and Mn(III)TBAP on quantitation of superoxide using hydroethidine. Free Radic. Biol. Med. 41, 1050–1057 (2006).
Ross, M.F. et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry Mosc. 70, 222–230 (2005).
Troiano, L. et al. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat. Protoc. 2, 2719–2727 (2007).
Davey, G.P., Tipton, K.F. & Murphy, M.P. Uptake and accumulation of 1-methyl-4-phenylpyridinium by rat liver mitochondria measured using an ion-selective electrode. Biochem. J. 288, 439–443 (1992).
Zimmerman, M.C., Oberley, L.W. & Flanagan, S.W. Mutant SOD1-induced neuronal toxicity is mediated by increased mitochondrial superoxide levels. J. Neurochem. 102, 609–618 (2007).
Mukhopadhyay, P., Rajesh, M.,, Haskó, G., Hawkins, B.J., Madesh, M., & Pacher, P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2, 2295–2301 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Mike Janes is an employee of Invitrogen, Inc.
Rights and permissions
About this article
Cite this article
Robinson, K., Janes, M. & Beckman, J. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc 3, 941–947 (2008). https://doi.org/10.1038/nprot.2008.56
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.56
- Springer Nature Limited
This article is cited by
-
Mitochondrial Dysfunction due to Novel COQ8A Variation with Poor Response to CoQ10 Treatment: A Comprehensive Study and Review of Literatures
The Cerebellum (2024)
-
Perturbing tumor cell metabolism with a Ru(II) photo-redox catalyst to reverse the multidrug resistance of lung cancer
Science China Chemistry (2023)
-
Longitudinal characterization of cerebral hemodynamics in the TgF344-AD rat model of Alzheimer’s disease
GeroScience (2023)
-
Oxygen radicals and cytoplasm zoning in growing lily pollen tubes
Plant Reproduction (2021)
-
Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction
GeroScience (2020)