Abstract
Batteries using lithium (Li) metal as anodes are considered promising energy storage systems because of their high energy densities. However, safety concerns associated with dendrite growth along with limited cycle life, especially at high charge current densities, hinder their practical uses. Here we report that an optimal amount (0.05 M) of LiPF6 as an additive in LiTFSI–LiBOB dual-salt/carbonate-solvent-based electrolytes significantly enhances the charging capability and cycling stability of Li metal batteries. In a Li metal battery using a 4-V Li-ion cathode at a moderately high loading of 1.75 mAh cm−2, a cyclability of 97.1% capacity retention after 500 cycles along with very limited increase in electrode overpotential is accomplished at a charge/discharge current density up to 1.75 mA cm−2. The fast charging and stable cycling performances are ascribed to the generation of a robust and conductive solid electrolyte interphase at the Li metal surface and stabilization of the Al cathode current collector.
Similar content being viewed by others
Lithium (Li) metal is regarded as the ultimate anode for energy storage systems because of its ultrahigh specific capacity of 3,860 mAh g−1, a very low redox potential (−3.040 V versus standard hydrogen electrode) and a small gravimetric density of 0.534 g cm−3. Secondary Li metal batteries (LMBs) have been extensively studied in the past four decades, and received increasing attention recently because of the growing needs for high-energy-density batteries1,2,3,4. However, technical challenges such as unsatisfied coulombic efficiency and dendritic Li growth impede the successful deployment of secondary LMBs. Recently, extensive work has been dedicated to Li metal protection, including the application of polymer or solid-state electrolytes5,6,7, ionic liquids8,9,10, concentrated electrolytes or additives,11,12 protective layers13,14, interlayers between Li and separator15,16, nanoscale design17, selective deposition3, Li/reduced graphene oxide composites4, and others.
Most applications of LMBs demand high-power performance—that is, fast charging and discharging capabilities. Fast discharging at a 2–4 mA cm−2 current density has been demonstrated to be beneficial for improving the cyclability of LMBs because of the stabilized Li metal anode protected by a transient liquid layer of a highly concentrated Li+ ion electrolyte18. However, fast charging at >0.6 ∼ 0.7 mA cm−2 is detrimental to LMBs using regular LiPF6/carbonate-solvent electrolytes because the aggravated evolution of dendritic Li along with the serious attack by the electrolyte results in the fast generation of a fairly resistive layer (that is, the solid electrolyte interphase (SEI)) on the Li metal surface, thus dramatically shortening the lifespan of LMBs19,20. Although a limited improvement in the charging rate of LMBs using carbonate solvent-based electrolytes has been achieved by using a dual-salt electrolyte consisting of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) together with lithium bis(oxalato)borate (LiBOB)20, the stability and the charge rate of these batteries are still far below those required for high-power applications. Therefore, further enhancing the charging capability and the cycling performance is critically important for enabling successful applications of rechargeable LMBs.
Here we report that significantly improved charging capability and cycling stability of LMBs using a LiNi0.4Mn0.4Co0.2O2 (NMC, 1.75 mAh cm−2) cathode can be achieved via manipulating the lithium salt chemistry in the electrolyte to generate a highly conductive SEI on Li metal. An optimal additive level (0.05 M) of LiPF6 can greatly alter the interfacial reactions between Li metal and dual-salt electrolyte containing LiTFSI and LiBOB in carbonate solvents. The capacity retention of moderately high areal-capacity Li||NMC batteries could be significantly improved to >97% after 500 cycles at 1.75 mA cm−2.
Electrochemical properties
The electrochemical properties of Li||NMC batteries using three different electrolytes are presented in Fig. 1. Two formation cycles under about 0.175 mA cm−2 (C/10 rate) were conducted prior to the subsequent cycling at higher charge/discharge current densities. During the formation cycles, all batteries exhibit almost the same charge/discharge voltage profiles and deliver a similar specific capacity ( ∼160 mAh g−1) that corresponds to a capacity loading of about 1.75 mAh cm−2 (Supplementary Fig. 1).
a, Li||NMC battery cycling performance with various electrolytes in EC–EMC solvent mixture at the same charge and discharge current density of 1.75 mA cm−2 after three formation cycles at 0.175 mA cm−2 under 30 ∘C. b–d, Voltage curves as a function of cycle number of Li||NMC batteries using conventional LiPF6 electrolyte (b), dual-salt (LiTFSI–LiBOB) electrolyte (c) and 0.05 M LiPF6-added dual-salt electrolyte (d).
Here it is worth mentioning two key parameters—the cathode areal loading and the charge current density—that dictate the capacity fading mechanism of LMBs. A higher cathode loading indicates more utilization of Li in each deposition/stripping process, resulting in more parasitic side reactions, and thus faster capacity fading (Supplementary Fig. 2a). In this work, the deposition and stripping of a large amount of Li (1.75 mAh cm−2) in each cycle allow us to reliably evaluate the electrolyte impact on the charging capability of LMBs. On the other hand, a higher charge current density leads to the quicker corrosion of Li metal19, and therefore leads to faster capacity fading of Li||NMC batteries. Using the conventional electrolyte, that is, 1.0 M LiPF6 in ethylene carbonate (EC) and ethyl methyl carbonate (EMC), the normal operation of the Li||NMC battery is sustained at 0.58 mA cm−2 (C/3) for charging, exhibiting a capacity retention of 86.3% at the 500th cycle (Supplementary Fig. 2b). However, with fast charge at 1.75 mA cm−2, the Li||NMC battery experiences an abrupt capacity drop after only about 60 cycles (Fig. 1a). This can be attributed to the quick accumulation of a fairly resistive surface film on the Li metal surface19. The consequence is a dramatic increase of cell impedance and electrode overpotential (Fig. 1b), leading to battery failure at an early stage.
In comparison, the dual-salt (LiTFSI–LiBOB) electrolyte has better compatibility with Li metal anodes. The corresponding Li||NMC battery shows greatly improved cycle life even at a high charge current density (Fig. 1a). The battery could survive up to 450 cycles prior to sharp capacity drop. However, this battery shows continuous capacity fading and an increased cell overpotential during cycling (Fig. 1c), suggesting the resistive nature of the SEI layer formed in this electrolyte. The capacity retention is only 74.5% after 450 cycles. Surprisingly, with the addition of 0.05 M LiPF6 as an additive to this dual-salt electrolyte, the Li||NMC battery shows significantly enhanced cycling performance. The battery still retains a high specific capacity of 140 mAh g−1 at the 500th cycle at 1.75 mA cm−2, corresponding to 97.1% capacity retention. More importantly, there is only a minimal increase of cell overpotential during 500 cycles (Fig. 1d), indicating that the Li/electrolyte interphase layer is highly conductive for Li+ ion transportation as compared to those formed in other two electrolytes. To our best knowledge, the performance is the best ever reported for LMBs at such a high charge current density and high positive electrode loading. The fast charging capability of LMBs presented here far exceeds those using concentrated electrolytes and ionic-liquid-based electrolytes8,12.
The electrochemical performance of Li||NMC batteries was also tested at 3.5 mA cm−2 (2C rate) and 5.25 mA cm−2 (3C rate) (Supplementary Fig. 3). At these high current densities, significant enhancement in cycle life is again achieved for Li||NMC batteries employing the LiPF6-added dual-salt electrolyte. For instance, when cycling at 3.5 mA cm−2, batteries using conventional LiPF6 solution and the additive-free dual-salt electrolyte reach end of life (EOL, 80% capacity retention) at 55 and 92 cycles, respectively, while the battery using the LiPF6-added dual-salt electrolyte can survive more than 200 cycles before the EOL.
In addition, the charging/discharging performance at different C rates was also evaluated, using two different protocols: that is, charge at the constant C/5 rate but discharge at an increasing C rate; and charge at an increasing C rate but discharge with constant C/5 rate. Under different protocols, Li||NMC batteries using the LiPF6-added dual-salt electrolyte always show superior charging and discharging capability as compared to the additive-free dual-salt electrolyte and the conventional LiPF6 electrolyte (Fig. 2).
It is well known that the conventional LiPF6/EC–EMC electrolyte results in a poor SEI layer forming, which contains a large amount of resistive decomposition products of LiF and Li2CO3, as well as other inorganic and organic by-products. Therefore, to reduce the formation of highly resistive LiF, the content of LiPF6 additive in the dual-salt electrolyte is critical. As presented in Fig. 3a,b, addition of 0.02 M of LiPF6 is able to considerably enhance the cycle life of Li||NMC batteries at 1.75 mA cm−2. Increasing the amount of LiPF6 to 0.05 M affords the maximized cycling stability. However, further increase of LiPF6 content to 0.1 M and 0.2 M leads to degradation of battery performance, showing fast capacity drop at earlier cycles. The reason is that excessive LiPF6 additive (0.1 M and 0.2 M) actually makes the dual-salt electrolytes behave more like the pure LiPF6-based electrolyte—that is, more LiPF6 will dominate the SEI formation and accelerate the electrolyte consumption, as occurs in the conventional LiPF6/carbonate electrolytes. The optimal LiPF6 content that is beneficial to dual-salt electrolytes of LiTFSI–LiBOB is ∼0.05 M.
a,b, Initial charge/discharge profiles at 0.175 mA cm−2 (a) and cycling performance (b) of Li||NMC batteries with dual-salt electrolytes containing various proportions of LiPF6 additive at 1.75 mA cm−2. c, Cycling performance of Li||NMC batteries using different electrolytes at a high temperature (60 ∘C). d, Cycling stability of Li||NMC battery using the 0.05 M LiPF6-added dual-salt electrolyte cycled with 0.58 mA cm−2 for charge and 1.75 mA cm−2 for discharge at 30 ∘C.
The performance of Li||NMC batteries using different electrolytes was further investigated at an elevated temperature (60 ∘C). Like the 30 ∘C results, batteries using the conventional LiPF6 electrolyte as well as the dual-salt electrolyte show inferior cyclability compared to the optimal LiPF6-added LiTFSI–LiBOB dual-salt electrolyte (Fig. 3c). An increase in the testing temperature will drastically accelerate the parasitic reactions of Li metal with electrolyte. This will cause the formation of more components in the SEI film and accelerate the degeneration of Li metal, which in turn results in fast capacity loss. Therefore, Li||NMC batteries show a shortened cycle life at 60 ∘C, regardless of the electrolyte used (Fig. 3c). The batteries using the conventional LiPF6 electrolyte and the additive-free dual-salt electrolyte terminate stable cycling at only 30 and 213 cycles, respectively. However, the battery using the LiPF6-added dual-salt electrolyte can still deliver 147 mAh g−1 at the 400th cycle at 1.75 mA cm−2, which corresponds to 90.0% of its initial capacity. All of these superior electrochemical performance results demonstrate that the optimal LiPF6-added dual-salt electrolyte has better compatibility with Li metal anodes both in fast charging processes and at elevated temperatures.
Additionally, the cycle life of a Li||NMC battery using the 0.05 M LiPF6-added dual-salt electrolyte could be further extended when a lower charge current density is used. At room temperature, a very limited capacity fade after 800 cycles (that is, 0.004375% fade per cycle) together with a high cycling efficiency over 99% was achieved for the Li||NMC battery cycled by charging under 0.58 mA cm−2 but discharging under 1.75 mA cm−2 (Fig. 3d). Benefiting from its excellent stability toward Li metal, the optimal LiPF6-added dual-salt electrolyte could be regarded as an appealing electrolyte for practical applications in rechargeable LMBs. Nevertheless, the average coulombic efficiency of Li metal anode itself ( ∼90.6%) is still not satisfactory due to the instability of carbonate solvents towards Li metal (Supplementary Fig. 4), which requires further improvement in future investigations.
Morphology evolution of Li metal anode during cycling
One question that may be raised regarding these excellent performance results is how the additive level of LiPF6 could greatly enhance the charging capability and the cyclability of LMBs. Structurally, NMC positive electrodes are fairly stable when charged to 4.3 V because of the limited Li+ ions (x < 0.6 in Li1−xMO2) utilized in each charge/discharge process. The NMC electrodes exhibit almost unchanged X-ray diffraction (XRD) patterns after cycling in different electrolytes (Supplementary Fig. 5). To further demonstrate the stability of the surface film formed on positive electrodes, cycled NMC electrodes were retrieved (Supplementary Fig. 6a), reassembled into new batteries with fresh Li metal foils using the regular LiPF6 electrolyte, and cycled again at 1.75 mA cm−2. All the reassembled batteries show an abrupt capacity drop at about 60–70 cycles (Supplementary Fig. 6b), revealing that the SEI layer, if any, formed on NMC electrodes could not produce the decisive role in improving the cycling stability of LMBs at high current densities. The NMC electrode pre-cycled in the LiPF6-added LiTFSI–LiBOB electrolyte does not show any advantage in cycle life. The results confirm with confidence that deterioration of the Li metal anode is the most significant consideration dominating the cycling stability of LMBs cycled at high charge current densities.
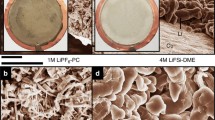
To obtain more insightful information, a scanning electron microscope (SEM) was used to scrutinize the morphologies of Li metal anodes after cycling, as displayed in Fig. 4a–f. SEM images of a fresh Li metal foil are presented in Supplementary Fig. 7 as a reference. Noticeable differences are evident on the cycled Li metal, depending on various electrolytes used. The LiPF6/EC–EMC electrolyte leads to substantial cracking (Supplementary Fig. 8a,a1,a2) and significant exacerbation of the bulk Li metal anode (Fig. 4a). Although sporadic needle-like dendritic Li is discernible from the Li metal surface (Fig. 4d), the core part of the bulk Li is constructed by loose Li with nano-to-micro particle sizes. This phenomenon indicates that the poor quality of this SEI layer could not shield the bulk Li against electrolyte attack. Consequently, the cell impedance increases quickly during cycling, as confirmed by the electrochemical impedance spectra (EIS) (see Fig. 4g–i). The electrolyte resistance and the interfacial resistance were estimated from the impedance spectra and summarized in Supplementary Table 1. In particular, the obvious increase in electrolyte resistance, as pointed out with a red arrow in Fig. 4i, suggests depletion of the electrolyte components, which affects the transport of Li+ ions and terminates the operation of LMBs.
a–f, Cross-section views (a–c) and top views (d–f) of cycled Li electrodes recovered from Li||NMC batteries following 100 cycles using conventional LiPF6 electrolyte (a,d), dual-salt (LiTFSI–LiBOB) electrolyte (b,e) and 0.05 M LiPF6-added dual-salt electrolyte (c,f). The arrows in a–c indicate the different corrosion depth of the Li metal anode. g–i, Nyquist plots of Li||NMC batteries using conventional electrolyte, dual-salt electrolyte and 0.05 M LiPF6-added dual-salt electrolyte at different stages of cycling. i, An enlarged view of the high-frequency region labelled by the red rectangle in h.
In contrast, the Li metal anodes retrieved from the two dual-salt electrolytes exhibit much different morphologies. The major part of the bulk Li metal anode is still well maintained (Fig. 4b,c), exhibiting a corrosion depth of about 30–50 μm. This surface layer sits only loosely on the top of bulk Li; it is susceptible to external pressure and could be easily peeled off from the bulk Li, especially when cycling the Li anode in additive-free dual-salt electrolyte (Fig. 4b). From the top view (Fig. 4e,f), it is found that the original solid Li metal surface has been transformed to fibrous Li with a length ranging from ∼10 to several tens μm, and a diameter of about 1 μm. Formation of fibrous Li is dictated by LiBOB21, while LiPF6 and LiTFSI mainly lead to the deposition of short needle-like Li (Supplementary Fig. 9).
For the additive-free dual-salt electrolyte, besides the fibrous Li formation, some nanosized dendritic Li has also been formed after cycling (Fig. 4e), which could increase the parasitic reactions between Li and electrolyte and cause more electrolyte decomposition and by-product formation. The SEI layer generated in the additive-free dual-salt electrolyte is fairly resistive, as indicated by the considerable increase in charge transfer resistance. In contrast, using LiPF6-added dual-salt electrolyte, the Li metal anode has only fibrous Li and some SEI layer skins left after Li stripping (Fig. 4f). Formation of fibrous Li is beneficial for enabling the high charging capability because the improved SEI layer as modified by the presence of LiPF6 additive leads to lower charge transfer resistance (Fig. 4h,i). The fast charge transfer nature of the SEI layer formed in the 0.05 M LiPF6-added dual-salt electrolyte enables the complete stripping of Li that is wrapped by SEI and mitigates accumulation of isolated or ‘dead’ Li.
SEI layer composition of cycled Li anode
X-ray photoelectron spectroscopy (XPS) was further employed to investigate the components of the SEI layer generated on cycled Li metal. For the Li metal cycled in the LiPF6-free dual-salt electrolyte, the SEI layer components include the carbonyl group ( ∼289.0 eV (O3)), polyether carbon ( ∼286.5 eV (H2O)), hydrocarbon, ( ∼285.0 eV (H2-CH2O)) in C 1s spectra (Fig. 5a–c), and the carbonyl ( ∼531.0 eV (C))/ether oxygen ( ∼533.0 eV (C--C)) in O 1s spectra (Fig. 5g–i)22,23. The dominating carbonyl groups could have originated from decompositions of both carbonate solvents and LiBOB salt anions. A significant quantity of Li carbides for example, LiCH2CH2OCO2Li from the reduction of EC, is obviously observed in this SEI, as indicated by the considerable carbon signal at ∼283.0–283.5 eV (refs 22,24). The decomposition products Li2O, LiF and BOx could also be confirmed in this SEI layer (Supplementary Fig. 10). The evolution of selected elements with depth profiling is illustrated in Supplementary Fig. 11. An SEI layer like this containing both organic and inorganic segments can hardly prevent Li metal from successive corrosion by the electrolyte. Meanwhile, an SEI layer that was produced in additive-free dual-salt electrolyte seems to be too fragile to protect Li, as evidenced by the earlier exposure of metallic Li after the same time period of XPS sputtering (Supplementary Fig. 10). The depletion of electrolyte and the relatively rapid corrosion of Li metal lead to continuous performance degradation (Figs 1 and 3).
a–l, XPS spectra of C 1s (a–f) and O 1s (g–l) for Li metal retrieved from Li||NMC batteries with dual-salt electrolyte (a–c,g–i) or using the LiPF6-added dual-salt electrolyte (d–f,j–l) after two formation cycles under 0.175 mA cm−2 and ten regular cycles under 1.75 mA cm−2. C 1s0 nm, C 1s10 nm, C 1s25 nm, and O 1s0 nm, O 1s10 nm, O 1s25 nm indicate the depths at which the C 1s or O 1s spectra were acquired. In the C 1s spectra, the fitted peaks in violet, magenta, orange, olive and yellow are related to polycarbonate, carbonyl group, polyether carbon, hydrocarbon, and lithium carbide species, respectively. In the O 1s spectra, the fitted peaks in violet, orange, olive and yellow are related to polycarbonate, polyether carbon, carbonyl group, and lithium oxides, respectively.
When the electrolyte is replaced with LiPF6-added dual-salt electrolyte, the Li metal surface is covered with an SEI layer of different components (Fig. 5d–f,j–l). The C 1s spectra show weak peaks with respect to carbides at 283.0–283.5 eV. Alternatively, extra C 1s peaks at 291.0–293.0 eV (Fig. 5d–f) and O 1s peaks at 534.0–536.0 eV (Fig. 5j–l) are observed. The result illustrates that the components of the SEI produced in LiPF6-added dual-salt electrolyte have higher binding energies than lithium carbonate and lithium alkyl carbonate have25,26, and they could be assigned to polycarbonates [poly(CO3)]22. These observations suggest that the LiPF6 additive helps induce the formation of polycarbonate species, which are more stable to protect Li metal against the electrolyte attack18. One possibility is that the trace amount of strong Lewis acids PF5 and POF3 derived from the additive level of LiPF6 (through the thermal decomposition of LiPF6 → LiF + PF5, and the hydrolysis of LiPF6+ H2O → LiF + 2HF + POF3) induces the polymerization of EC solvents. The SEI layer enriched with polycarbonate constituents could effectively mitigate the attack by the electrolyte, and may still permit prompt transport of Li+ ions and afford sustainable use of LMBs. In addition, the polycarbonate moieties probably have relatively high molecular weights, so they can act in the role of polymer binder to hold other ionically conductive SEI components together and intimately stick to the bulk Li metal anode (Fig. 4cand Supplementary Fig. 8c1,c2).
To gain insight into what functionalities begin to form at higher LiPF6 additive concentrations, we also performed XPS measurements on the Li metal anodes cycled in 0.1 M and 0.2 M LiPF6-added dual-salt electrolytes (Supplementary Fig. 12). Raising the content of LiPF6 additive to 0.1 M and 0.2 M in the dual-salt electrolyte not only reduces the formation of poly(CO3) constituents but also increases the formation of inorganic components dominated with carbonates and Li2O. Such an SEI enriched with inorganic carbonates and Li2O could not provide enough mechanical strength to maintain the SEI layer from breakdown, so it cannot effectively protect the Li metal against corrosion from the electrolyte. Therefore, higher LiPF6 additive concentrations again accelerate the consumption of electrolyte, similar to what occurs in the conventional LiPF6 electrolyte. Therefore, the batteries with 0.1 M and 0.2 M LiPF6-added dual-salt electrolytes show fast capacity degradation after a few stable cycles (Fig. 3b).
Electrolyte and SEI layer evolution
Thermodynamically, the electrochemical reduction potential of a lithium salt or solvent is closely related to its lowest unoccupied molecular orbital (LUMO) energy, a lower value of which typically indicates that a chemical is reduced at a higher voltage. The LUMO energy values obtained by molecular dynamics (MD) simulations shown in Fig. 6a demonstrate that the reduction voltage decreases in the following order: LiBOB ≫ LiTFSI >∼LiPF6 ≫ EC > EMC. LiBOB could be easily reduced to form an SEI film and hence dictates the morphology of newly deposited Li (Supplementary Fig. 9). Cyclic voltammetry (CV) analysis (Supplementary Fig. 13) is consistent with the LUMO energies, demonstrating that the pure LiBOB shows a reduction peak with an onset voltage at about 1.1 V versus Li/Li+, while those for pure LiTFSI and LiPF6 electrolytes are hard to find. The reduction peak at 1.1 V for LiBOB in the LiTFSI–LiBOB electrolyte is greatly suppressed by the addition of LiPF6 additive, probably due to the superior SEI layer formed.
a, Highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies of the lithium salts (LiPF6, LiTFSI, LiBOB) and solvents (EC, EMC). The vertical lines indicate the electrochemical stability window of related lithium salts and solvents. b–e, EDS spectra of Li metal anodes after 100 cycles (b,d) and 500 cycles (c,e) in the dual-salt electrolytes without (b,c) and with (d,e) 0.05 M LiPF6 additive. Insets in b–e are the percentages of elements identified in cycled Li metal anodes. f, Schematic illustration demonstrating that a LiPF6 additive in LiTFSI–LiBOB electrolyte prevents the Al corrosion and improves the stability of Li metal.
The XPS results, as shown in Supplementary Figs. 11 and 12, are also in good accordance with the above LUMO energies. It is surprising to find that the by-products from the decomposition of LiTFSI are in trace amounts, especially the sulfur (S)-containing species, which are hardly detectable in the SEI produced in the dual-salt electrolytes after ten cycles (Supplementary Fig. 10e,f). The LiF found in the SEI generated in the additive-free LiTFSI–LiBOB electrolyte is definitely from the decomposition of LiTFSI (Supplementary Fig. 10a). This may indicate that some of the S-containing species are soluble in the electrolytes. When LiPF6 is added, it seems that the decomposition of LiTFSI is suppressed and the LixPOyFz from the decomposition of LiPF6 and/or POF3 is obviously detected (Supplementary Fig. 10b). The evolution of the compositions, or specifically the corresponding elements, in the SEI layers during cycling has been further confirmed with energy dispersive X-ray spectroscopy (EDS) measurements (Fig. 6b,d). After 100 cycles, as compared to B (4.03%) and S (1.08%) found in the SEI layer formed in additive-free dual-salt electrolyte (Fig. 6b), lower contents of B (3.04%) and S (0.22%) elements are detected in the SEI formed in 0.05 M LiPF6-containing electrolyte (Fig. 6d), suggesting the robustness of the SEI layer and the mitigated reduction of LiTFSI and LiBOB salts in the presence of LiPF6 additive. The result is consistent with the CV data showing a lower current response and the suppression of the reduction peak of LiBOB during the negative scan (Supplementary Fig. 13f). After 500 cycles in the additive-free LiTFSI–LiBOB electrolyte, the Li surface film has a significant content of Al (Fig. 6c). This was unexpected, and indicates that LiTFSI–LiBOB electrolyte without LiPF6 additive still causes Al corrosion during long-term cycling, and the soluble corrosion product Al(TFSI)3 diffuses to and deposits onto the Li surface. In contrast, Al corrosion is completely suppressed in the presence of LiPF6 additive, as reflected by the absence of an Al signal in the EDS spectrum of the Li metal anode after 500 cycles in LiPF6-added LiTFSI–LiBOB electrolyte (Fig. 6e).
Based on the electrochemical performance and post-mortem analysis, we propose a functioning mechanism of LiPF6 additive in LiTFSI–LiBOB dual-salt electrolyte (Fig. 6f). On one hand, the LiPF6 additive is a critical piece in stabilizing Al foil and maintaining electrical connection with the active material. On the other hand, a small amount of LiPF6 additive greatly alters the nature of the SEI layer generated on the Li anode surface. LiPF6 additive chemically or electrochemically decomposed to form PF5, HF and POF3, which then act as catalysts and induce the formation of polycarbonates from the ring-opening polymerization of EC molecules (Fig. 5d–f,j–l)27,28. The SEI layer produced in the LiPF6-added dual-salt electrolyte is highly conductive and has very limited effects on the electrode polarization (Fig. 1d and Supplementary Fig. 14), which could prevent the accumulation of isolated/‘dead’ Li during each deposition/stripping cycle. In addition, the polycarbonates formed in the SEI layer are flexible, can efficiently cover the Li metal surface, reduce the side reactions, hold the isolated/‘dead’ Li particles tightly and adhere to the bulk Li anode, thus preventing the detachment of the SEI layer from the bulk Li metal. Therefore, the utilization of Li metal initiates at the surface and slowly propagates inwards (Fig. 4c). More importantly, there is no internal short circuit occurring with a charge current up to 5.25 mA cm−2. This high charging capability and the excellent cycle life validate the LiPF6-added LiTFSI–LiBOB dual-salt electrolyte as an appealing functional electrolyte for high-performance LMBs.
Conclusions
We discovered that an optimal additive amount (0.05 M) of LiPF6 in the LiTFSI–LiBOB/carbonate electrolytes could greatly enhance the charging performance and the cyclability of LMBs. Stable cycling along with a very limited increase of electrode overpotential has been achieved at current densities of 1.75 mA cm−2 and above with the electrode loading of 1.75 mAh cm−2. The LiPF6 additive not only plays a critical role in stabilizing the Al current collector, but more importantly also induces the generation of a robust and conductive SEI layer enriched with polycarbonate constituents that can effectively bond the isolated/‘dead’ Li with the bulk Li metal anode. The enhanced interfacial stability allows sustainable operation of LMBs at high charge current densities. These key findings offer deep insights to inspire revolutionary improvements in the performance of LMBs through manipulating the salt/solvent/additive chemistry of the electrolytes.
Methods
Materials.
Cathode NMC (LiNi0.4Mn0.4Co0.2O2) electrode laminates ( ∼10.8 mg active material cm−2) were supplied by the Cell Analysis, Modelling, and Prototyping (CAMP) Facility located at Argonne National Laboratory (ANL). The electrode laminates were punched into discs and further dried at ∼75 ∘C under vacuum for 12 h. Battery-grade LiPF6, LiTFSI, EC, and EMC were purchased from BASF. Battery-grade LiBOB was kindly provided by Chemetall for free. These chemicals were kept and handled in a glovebox (MBraun LABmaster) circulated with high-purity Ar gas ( <1 ppm O2 and <1 ppm H2O). The LiTFSI–LiBOB electrolyte consisting of 0.6 M LiTFSI plus 0.4 M LiBOB in the solvent mixture of EC and EMC (4:6 by wt) was blended inside the glovebox. LiPF6-added dual-salt electrolytes were prepared through the addition of required amounts of LiPF6 to the as-prepared dual-salt electrolyte. A control electrolyte (that is, 1.0 M LiPF6 in the same EC–EMC solvents) was prepared and evaluated for comparison.
Characterization.
Structural analysis of NMC electrodes before and after cycling was conducted with a Rigaku MiniFlex II XRD instrument using Cu Kα radiation run at 15 mA and 30 kV. XRD patterns were recorded from 10 ∼ 90∘ in 2θ with a scan speed of 0.1∘ per min. Morphology observations along with the EDS measurement of cycled Li metal were performed on a Helios focused ion beam SEM at 5.0 kV. For sample preparation, the cycled Li metal samples were soaked in pure EMC for 1 h and then cleaned with pure EMC many times to eliminate remaining electrolytes, and finally dried under vacuum. The cross-sections of Li metal were obtained by using a razor blade to cut the Li metal foils. The XPS testing was carried out using the Physical Electronics Quantera scanning X-ray microprobe, which was outfitted with a monochromatic Al Kα X-ray source (1,486.7 eV) for excitation. To avoid side reactions or electrode contamination with ambient oxygen and moisture, Li metal samples were transported from the glovebox to the SEM and XPS instruments in a hermetically sealed container protected by Ar gas.
Electrochemical testing.
Charge/discharge performances were measured using CR2032 coin-type batteries. LMBs were constructed using an NMC positive electrode, a foil of Li metal anode, one piece separator (Celgard 2500), and the prepared electrolyte (100 μl in each battery). Cycling and rate performance tests were carried out with constant current and constant voltage mode using battery testers (Arbin BT-2000). 30 ∘C or 60 ∘C environmental chambers were used to keep the temperature stable during the battery performance evaluation. All the Li||NMC batteries were tested between 2.7 and 4.3 V. When the voltage reached 4.3 V, a constant voltage charge process (4.3 V) was applied until the charge current decayed to 0.175 mA cm−2 (C/10). 1C is equal to 160 mA g−1 or 1.75 mA cm−2, depending on the active mass loading of NMC cathode material. Current densities of 0.58, 1.75, 3.50 and 5.25 mA cm−2 were used for cycling performance testing of Li||NMC batteries so as to assess the charging capability of the studied electrolytes. EIS was executed on a 1255B Solartron frequency response analyser coupled with a 1287 potentiostat/galvanostat electrochemical interface (±10 mV perturbation and 105 ∼ 10−3 Hz frequency range).
MD calculations.
The highest occupied molecular orbital (HOMO) and LUMO energies of EC, EMC, LiPF6, LiBOB and LiTFSI were calculated by density functional theory using the Gaussian 09 software29. Geometry optimization simulations were carried out using the basis set of 6–311 + +G(d,p) in conjunction with the functional B3LYP30,31.
Data availability.
The supporting data for the included charts/graphs within this paper, as well as other findings from this study, are available from the corresponding author upon reasonable request.
Additional Information
How to cite this article: Zheng, J. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017).
References
Lu, Y., Tu, Z. & Archer, L. A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Yan, K. et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 16010 (2016).
Lin, D. et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotech. 11, 626–632 (2016).
Khurana, R., Schaefer, J. L., Archer, L. A. & Coates, G. W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014).
Zhou, D. et al. SiO2 Hollow nanosphere-based composite solid electrolyte for lithium metal batteries to suppress lithium dendrite growth and enhance cycle life. Adv. Energy Mater. 6, 201502214 (2016).
Choudhury, S., Mangal, R., Agrawal, A. & Archer, L. A. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat. Commun. 6, 10101 (2015).
Basile, A., Bhatt, A. I. & O’Mullane, A. P. Stabilizing lithium metal using ionic liquids for long-lived batteries. Nat. Commun. 7, 11794 (2016).
Bhatt, A. I., Best, A. S., Huang, J. & Hollenkamp, A. F. Application of the N-propyl-N-methyl-pyrrolidinium bis(fluorosulfonyl)imide RTIL containing lithium bis(fluorosulfonyl)imide in ionic liquid based lithium batteries. J. Electrochem. Soc. 157, A66–A74 (2010).
Basile, A., Hollenkamp, A. F., Bhatt, A. I. & O’Mullane, A. P. Extensive charge–discharge cycling of lithium metal electrodes achieved using ionic liquid electrolytes. Electrochem. Commun. 27, 69–72 (2013).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Li, N.-W., Yin, Y.-X., Yang, C.-P. & Guo, Y.-G. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 28, 1853–1858 (2016).
Kazyak, E., Wood, K. N. & Dasgupta, N. P. Improved cycle life and stability of lithium metal anodes through ultrathin atomic layer deposition surface treatments. Chem. Mater. 27, 6457–6462 (2015).
Yan, K. et al. Ultrathin two-dimensional atomic crystals as stable interfacial layer for improvement of lithium metal anode. Nano Lett. 14, 6016–6022 (2014).
Cheng, X.-B. et al. Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv. Mater. 28, 2888–2895 (2016).
Zheng, G. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotech. 9, 618–623 (2014).
Zheng, J. et al. Highly stable operation of lithium metal batteries enabled by the formation of a transient high-concentration electrolyte layer. Adv. Energy Mater. 6, 201502151 (2016).
Lu, D. et al. Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv. Energy Mater. 5, 1400993 (2015).
Xiang, H. et al. Enhanced charging capability of lithium metal batteries based on lithium bis(trifluoromethanesulfonyl)imide-lithium bis(oxalato)borate dual-salt electrolytes. J. Power Sources 318, 170–177 (2016).
Ding, F. et al. Effects of carbonate solvents and lithium salts on morphology and coulombic efficiency of lithium electrode. J. Electrochem. Soc. 160, A1894–A1901 (2013).
Ota, H., Sakata, Y., Inoue, A. & Yamaguchi, S. Analysis of vinylene carbonate derived SEI layers on graphite anode. J. Electrochem. Soc. 151, A1659–A1669 (2004).
Chan, C. K., Ruffo, R., Hong, S. S. & Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Sources 189, 1132–1140 (2009).
Aurbach, D. et al. On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries. Electrochim. Acta 47, 1423–1439 (2002).
Dedryvère, R. et al. Characterization of lithium alkyl carbonates by X-ray photoelectron spectroscopy: experimental and theoretical study. J. Phys. Chem. B 109, 15868–15875 (2005).
Eshkenazi, V., Peled, E., Burstein, L. & Golodnitsky, D. XPS analysis of the SEI formed on carbonaceous materials. Solid State Ion. 170, 83–91 (2004).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Sloop, S. E., Pugh, J. K., Wang, S., Kerr, J. B. & Kinoshita, K. Chemical reactivity of PF5 and LiPF6 in ethylene carbonate/dimethyl carbonate solutions. Electrochem. Solid-State Lett. 4, A42–A44 (2001).
Frisch, M. J. et al. Gaussian 09 (Gaussian, 2009).
Boys, S. F. & Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy (DOE) through the Advanced Battery Materials Research (BMR) Program under contract no. DE-AC02-05CH11231. W.X. also thanks the Battery500 Consortium for the partial support through the BMR Program. Microscopy as well as spectroscopy characterizations were performed in the William R. Wiley Environmental Molecular Sciences Laboratory, a National Scientific User Facility sponsored by DOE’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for the DOE under contract no. DE-AC05-76RLO1830. The NMC electrodes were produced at the US DOE’s CAMP Facility, ANL. The CAMP Facility is fully supported by the DOE VTO within the core funding of the Applied Battery Research (ABR) for Transportation Program.
Author information
Authors and Affiliations
Contributions
J.Z., W.X. and J.-G.Z. initiated this research and designed the experiments. B.J.P. prepared and provided the standard NMC electrodes. J.Z. performed the electrochemical measurements with assistance from S.J., as well as the SEM observations. M.H.E. and D.M. performed XPS measurements and molecular dynamics simulations, respectively. J.Z., J.-G.Z., and W.X. prepared this manuscript with input from other co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–14, Supplementary Table 1, Supplementary References. (PDF 1940 kb)
Rights and permissions
About this article
Cite this article
Zheng, J., Engelhard, M., Mei, D. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat Energy 2, 17012 (2017). https://doi.org/10.1038/nenergy.2017.12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2017.12
- Springer Nature Limited
This article is cited by
-
Manipulating the diffusion energy barrier at the lithium metal electrolyte interface for dendrite-free long-life batteries
Nature Communications (2024)
-
Lithium-induced graphene layer containing Li3P alloy phase to achieve ultra-stable electrode interface for lithium metal anode
Rare Metals (2024)
-
Exfoliated few-layered graphite anode with broadened delithiation voltage plateau and fast charging performance for lithium-ion batteries
Journal of Solid State Electrochemistry (2024)
-
Extreme fast charging of commercial Li-ion batteries via combined thermal switching and self-heating approaches
Nature Communications (2023)
-
An inorganic-rich but LiF-free interphase for fast charging and long cycle life lithium metal batteries
Nature Communications (2023)