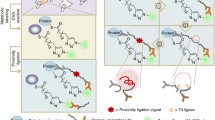
Abstract
Hundreds of human proteins are modified by reversible palmitoylation of cysteine residues (S-palmitoylation), but the regulation of depalmitoylation is poorly understood. Here, we develop 'depalmitoylation probes' (DPPs), small-molecule fluorophores, to monitor the endogenous activity levels of 'erasers' of S-palmitoylation, acylprotein thioesterases (APTs). Live-cell analysis with DPPs reveals rapid growth-factor-mediated inhibition of the depalmitoylation activity of APTs, exposing a novel regulatory mechanism of dynamic lipid signaling.


Similar content being viewed by others
References
Linder, M.E. & Deschenes, R.J. Nat. Rev. Mol. Cell Biol. 8, 74–84 (2007).
Eisenberg, S. et al. Biochem. Soc. Trans. 41, 79–83 (2013).
Topinka, J.R. & Bredt, D.S. Neuron 20, 125–134 (1998).
Chan, P. et al. Nat. Chem. Biol. 12, 282–289 (2016).
Peng, T., Thinon, E. & Hang, H.C. Curr. Opin. Chem. Biol. 30, 77–86 (2016).
Hernandez, J.L., Majmudar, J.D. & Martin, B.R. Curr. Opin. Chem. Biol. 17, 20–26 (2013).
Zheng, B. et al. J. Am. Chem. Soc. 135, 7082–7085 (2013).
Verkruyse, L.A. & Hofmann, S.L. J. Biol. Chem. 271, 15831–15836 (1996).
Long, J.Z. & Cravatt, B.F. Chem. Rev. 111, 6022–6063 (2011).
Lin, D.T. & Conibear, E. eLife 4, e11306 (2015).
Rocks, O. et al. Cell 141, 458–471 (2010).
El-Husseini Ael, D. et al. Cell 108, 849–863 (2002).
Ponimaskin, E. et al. J. Neurosci. 28, 8897–8907 (2008).
Zhang, M.M., Tsou, L.K., Charron, G., Raghavan, A.S. & Hang, H.C. Proc. Natl. Acad. Sci. USA 107, 8627–8632 (2010).
Kong, E. et al. J. Biol. Chem. 288, 9112–9125 (2013).
Davda, D. & Martin, B.R. MedChemComm 5, 268–276 (2014).
Dekker, F.J. et al. Nat. Chem. Biol. 6, 449–456 (2010).
Rusch, M. et al. Angew. Chem. Int. Edn Engl. 50, 9838–9842 (2011).
Adibekian, A. et al. J. Am. Chem. Soc. 134, 10345–10348 (2012).
Görmer, K. et al. ChemBioChem 13, 1017–1023 (2012).
Creaser, S.P. & Peterson, B.R. J. Am. Chem. Soc. 124, 2444–2445 (2002).
Lee, J.H., Lim, C.S., Tian, Y.S., Han, J.H. & Cho, B.R. J. Am. Chem. Soc. 132, 1216–1217 (2010).
Dekker, F.J. & Hedberg, C. Bioorg. Med. Chem. 19, 1376–1380 (2011).
Paulsen, C.E. & Carroll, K.S. Chem. Rev. 113, 4633–4679 (2013).
Lin, H., Su, X. & He, B. ACS Chem. Biol. 7, 947–960 (2012).
Sauers, R.R., Husain, S.N., Piechowski, A.P. & Bird, G.R. Dyes Pigm. 8, 35–53 (1987).
Smith, G.A., Metcalfe, J.C. & Clarke, S.D. J. Chem. Soc. Perkin Trans. 2 1993, 1195–1204 (1993).
Acknowledgements
This work was supported by the University of Chicago, the National Institute of General Medical Sciences of the National Institutes of Health (R35 GM119840) to B.C.D., the University of Chicago Medicine Comprehensive Cancer Center (P30CA14599), and a “Catalyst Award” to B.C.D. from the Chicago Biomedical Consortium, with support from the Searle Funds at The Chicago Community Trust. We thank J. Pu (University of Chicago) and D. Dammeier (University of Chicago) for technical assistance, and C. He (University of Chicago), A. Mukherje (National Institutes of Health), Y. Krishnan (University of Chicago), and J. Lewis (University of Chicago) for supplying materials and equipment.
Author information
Authors and Affiliations
Contributions
R.S.K. and P.D.E. synthesized all compounds in the paper. R.S.K. performed all analytical measurements, in vitro assays, and cell culture experiments. R.S.K. and B.C.D. designed experimental strategies and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–17. (PDF 4117 kb)
Supplementary Note
Synthetic Procedures. (PDF 1604 kb)
Rights and permissions
About this article
Cite this article
Kathayat, R., Elvira, P. & Dickinson, B. A fluorescent probe for cysteine depalmitoylation reveals dynamic APT signaling. Nat Chem Biol 13, 150–152 (2017). https://doi.org/10.1038/nchembio.2262
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2262
- Springer Nature America, Inc.
This article is cited by
-
Mechanisms and functions of protein S-acylation
Nature Reviews Molecular Cell Biology (2024)
-
The Elk-3 target Abhd10 ameliorates hepatotoxic injury and fibrosis in alcoholic liver disease
Communications Biology (2023)
-
Synthesis and Investigation of Derivatives of 1,8-Naphthalimide with a Red Emission via an Aromatic Nucleophilic Substitution Reaction
Journal of Fluorescence (2022)
-
Palmitoylation is required for TNF-R1 signaling
Cell Communication and Signaling (2019)
-
ABHD10 is an S-depalmitoylase affecting redox homeostasis through peroxiredoxin-5
Nature Chemical Biology (2019)