Abstract
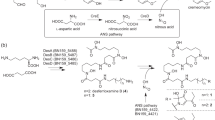
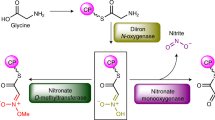
Although some diazo compounds have bioactivities of medicinal interest, little is known about diazo group formation in nature. Here we describe an unprecedented nitrous acid biosynthetic pathway responsible for the formation of a diazo group in the biosynthesis of the ortho-diazoquinone secondary metabolite cremeomycin in Streptomyces cremeus. This finding provides important insights into the biosynthetic pathways not only for diazo compounds but also for other naturally occurring compounds containing nitrogen-nitrogen bonds.


Similar content being viewed by others
Accession codes
References
Doyle, M.P., McKervey, M.A. & Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds (Wiley, New York, 1998).
Blair, L.M. & Sperry, J. J. Nat. Prod. 76, 794–812 (2013).
Itŏ, S., Matsuya, T., Omura, S., Otani, M. & Nakagawa, A. J. Antibiot. (Tokyo) 23, 315–317 (1970).
Nihei, Y. et al. J. Antibiot. (Tokyo) 46, 900–907 (1993).
Nawrat, C.C. & Moody, C.J. Nat. Prod. Rep. 28, 1426–1444 (2011).
Gould, S.J. Chem. Rev. 97, 2499–2510 (1997).
Bergy, M.E. & Pyke, T.R. US patent 3,350,269 (1967).
McGuire, J.N., Wilson, S.R. & Rinehart, K.L. J. Antibiot. (Tokyo) 48, 516–519 (1995).
Suzuki, H., Ohnishi, Y., Furusho, Y., Sakuda, S. & Horinouchi, S. J. Biol. Chem. 281, 36944–36951 (2006).
Smanski, M.J. et al. Proc. Natl. Acad. Sci. USA 108, 13498–13503 (2011).
Wishart, D.S. et al. J. Biomol. NMR 6, 135–140 (1995).
Zhang, J., Ma, W. & Tan, H. Eur. J. Biochem. 269, 6302–6307 (2002).
Baxter, R.L., Hanley, A.B. & Chan, H.W.S. J. Chem. Soc. Chem. Commun. 12, 757–758 (1988).
Winkler, R. & Hertweck, C. Angew. Chem. Int. Edn. 44, 4083–4087 (2005).
Chanco, E., Choi, Y.S., Sun, N., Vu, M. & Zhao, H. Bioorg. Med. Chem. 22, 5569–5577 (2014).
Li, N., Korboukh, V.K., Krebs, C. & Bollinger, J.M. Jr. Proc. Natl. Acad. Sci. USA 107, 15722–15727 (2010).
Franke, J., Ishida, K., Ishida-Ito, M. & Hertweck, C. Angew. Chem. Int. Ed. 52, 8271–8275 (2013).
Turner, N.J. Curr. Opin. Chem. Biol. 15, 234–240 (2011).
Isobe, K. & Ohte, N. Microbes Environ. 29, 4–16 (2014).
Winter, J.M., Jansma, A.L., Handel, T.M. & Moore, B.S. Angew. Chem. Int. Ed. 48, 767–770 (2009).
Gould, S.J., Hong, S.T. & Carney, J.R. J. Antibiot. (Tokyo) 51, 50–57 (1998).
Janso, J.E. et al. Tetrahedron 70, 4156–4164 (2014).
Gao, J. et al. Angew. Chem. Int. Edn Engl. 53, 1334–1337 (2014).
Le Goff, G. & Ouazzani, J. Bioorg. Med. Chem. 22, 6529–6544 (2014).
Waldman, A.J., Pechersky, Y., Wang, P., Wang, J.X. & Balskus, E.P. ChemBioChem 16, 2172–2175 (2015).
Onaka, H., Taniguchi, S., Ikeda, H., Igarashi, Y. & Furumai, T. J. Antibiot. (Tokyo) 56, 950–956 (2003).
Noguchi, A., Kitamura, T., Onaka, H., Horinouchi, S. & Ohnishi, Y. Nat. Chem. Biol. 6, 641–643 (2010).
Fu, J. et al. Nat. Biotechnol. 30, 440–446 (2012).
Bibb, M.J., Janssen, G.R. & Ward, J.M. Gene 38, 215–226 (1985).
Saltzman, B.E. Anal. Chem. 26, 1949–1955 (1954).
Varley, L. & Moody, C. Synthesis 2008, 3601–3604 (2008).
Lin, Y.-M. & Miller, M.J. J. Org. Chem. 64, 7451–7458 (1999).
Acknowledgements
We thank A. Luzhetskyy (Helmholtz Institute for Pharmaceutical Research Saarland), H. Onaka (University of Tokyo) and Y. Zhang (Gene Bridges) for providing pKGLP2, pTOYAMAcos and GB05dir, respectively. This research was supported in part by a funding program for next-generation world-leading researchers from the Bureau of Science, Technology and Innovation Policy, Cabinet Office, Government of Japan (GS006 to Y.O.), Grant-in-Aid for Young Scientists (B) (25850048 to Y.K.) and NC-CARP project from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (to Y.K. and Y.O.).
Author information
Authors and Affiliations
Contributions
Y.S. designed the study, performed experiments and wrote the manuscript. Y.K. designed the study, analyzed the data and wrote the manuscript. Y.O. directed the research, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1 and 2 and Supplementary Figures 1–26. (PDF 9033 kb)
Rights and permissions
About this article
Cite this article
Sugai, Y., Katsuyama, Y. & Ohnishi, Y. A nitrous acid biosynthetic pathway for diazo group formation in bacteria. Nat Chem Biol 12, 73–75 (2016). https://doi.org/10.1038/nchembio.1991
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1991
- Springer Nature America, Inc.
This article is cited by
-
Complete integration of carbene-transfer chemistry into biosynthesis
Nature (2023)
-
Changes in the membrane lipid composition of a Sulfurimonas species depend on the electron acceptor used for sulfur oxidation
ISME Communications (2022)
-
Molecular basis of enzymatic nitrogen-nitrogen formation by a family of zinc-binding cupin enzymes
Nature Communications (2021)
-
Biosynthesis of triacsin featuring an N-hydroxytriazene pharmacophore
Nature Chemical Biology (2021)
-
Nitric oxide as a source for bacterial triazole biosynthesis
Nature Communications (2020)