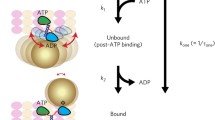
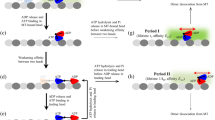
Abstract
A conventional kinesin molecule travels continuously along a microtubule in discrete 8-nm steps. This processive movement is generally explained by models in which the two identical heads of a kinesin move in a 'hand-over-hand' manner1,2,3,4. Here, we show that a single heterodimeric kinesin molecule (in which one of the two heads is mutated in a nucleotide-binding site) exhibits fast and slow (with the dwell time at least 10 times longer than that of the fast step) 8-nm steps alternately, presumably corresponding to the displacement by the wild-type and mutant heads, respectively. Our results provide the first direct evidence for models in which the roles of the two heads alternate every 8-nm step.


Similar content being viewed by others
References
Howard, J. The mechanics of force generation by kinesin. Biophys J. 68, S245–S253; S253–S255 (1995).
Block, S.M., Goldstein, L.S. & Schnapp, B.J. Bead movement by single kinesin molecules studied with optical tweezers. Nature 348, 348–352 (1990).
Hackney, D.D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc. Natl Acad. Sci. USA 91, 6865–6869 (1994).
Vale, R.D. & Milligan, R.A. The way things move: looking under the hood of molecular motor proteins. Science 288, 88–95 (2000).
Block, S.M. & Svoboda, K. Analysis of high resolution recordings of motor movement. Biophys J. 68, S2305–S2415 (1995).
Hua, W., Chung, J. & Gelles, J. Distinguishing inchworm and hand-over-hand processive kinesin movement by neck rotation measurements. Science 295, 844–848 (2002).
Kaseda, K., Higuchi, H. & Hirose, K. Coordination of kinesin's two heads studied with mutant heterodimers. Proc. Natl Acad. Sci. USA 99, 16058–16063 (2002).
Vale, R.D. Switches, latches, and amplifiers: common themes of G proteins and molecular motors. J. Cell Biol. 135, 291–302 (1996).
Cross, R.A. et al. The conformational cycle of kinesin. Phil. Trans R Soc. Lond B 355, 459–464 (2000).
Ma, Y.Z. & Taylor, E.W. Interacting head mechanism of microtubule-kinesin ATPase. J. Biol. Chem. 272, 724–730 (1997).
Kojima, H., Muto, E., Higuchi, H. & Yanagida, T. Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys J. 73, 2012–2022 (1997).
Forkey, J.N., Quinlan, M.E., Shaw, M.A., Corrie, J.E. & Goldman, Y.E. Three-dimensional structural dynamics of myosin V by single-molecule fluorescence polarization. Nature 422, 399–404 (2003).
Yildiz, A. et al. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science 300, 2061–2065 (2003).
Nishiyama, M., Muto, E., Inoue, Y., Yanagida, T. & Higuchi, H. Substeps within the 8-nm step of the ATPase cycle of single kinesin molecules. Nature Cell Biol. 3, 425–428 (2001).
Svoboda, K., Schmidt, C.F., Schnapp, B.J. & Block, S.M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993).
Svoboda, K. & Block, S.M. Force and velocity measured for single kinesin molecules. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994).
Higuchi, H., Muto, E., Inoue, Y. & Yanagida, T. Kinetics of force generation by single kinesin molecules activated by laser photolysis of caged ATP. Proc. Natl Acad. Sci. USA 94, 4395–4400 (1997).
Visscher, K. & Block, S.M. Versatile optical traps with feedback control. Methods Enzymol. 298, 460–489 (1998).
Acknowledgements
We thank L. Amos for comments on the manuscript. This work was aided by support from the Human Frontier Science Program (H.H. and K.H.) and the Japan Society for the Promotion of Science (K.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information, Fig. S1
Supplementary Information, Fig. S2 (PDF 96 kb)
Supplementary Information, Fig. S3
Rights and permissions
About this article
Cite this article
Kaseda, K., Higuchi, H. & Hirose, K. Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nat Cell Biol 5, 1079–1082 (2003). https://doi.org/10.1038/ncb1067
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1067
- Springer Nature Limited
This article is cited by
-
Optical Tweezers with Integrated Multiplane Microscopy (OpTIMuM): a new tool for 3D microrheology
Scientific Reports (2021)
-
The inchworm episode: Reconstituting the phenomenon of kinesin motility
European Journal for Philosophy of Science (2021)
-
Shaft Function of Kinesin-1’s α4 Helix in the Processive Movement
Cellular and Molecular Bioengineering (2019)
-
Design principles governing chemomechanical coupling of kinesin
Scientific Reports (2017)
-
The structural switch of nucleotide-free kinesin
Scientific Reports (2017)