Abstract
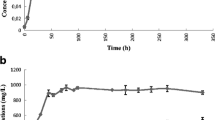
The alk genes from the catabolic OCT plasmid of Pseudomonas oleovorans, which encode the enzymes involved in the oxidation of n-alkanes to carboxylic acids, were introduced into E. coli W3110. The resulting recombinant converts n-octane in a two-liquid phase medium into the corresponding alkanoate and excretes this compound into the aqueous phase. The rate of octanoic acid production by the recombinant E. coli is equal to or better than the alkane oxidation rate of P. oleovorans, suggesting that two-liquid phase fermentations with E. coli might have future industrial applications.
Similar content being viewed by others
References
Chakrabarty, A.M. 1985. Genetically engineered microorganisms and their products in the oil service industries. TIBTECH 3: 32–38.
Ballard, D.G.H., Courtis, A., Shirley, I.M. and Taylor, S.C. 1983. A biotech route to polyphenylene. J. Chem. Soc. Chem. Comm. 634: 954–955.
Yi, Z.-H. and Rehm, H.J. 1982. Formation of α,ω-dodecanedioic acid and α,ω-tridecanedioic acid from differenet substrates by immobilized cells of a mutant of Candida tropicolis. Appl. Microbiol. Biotechnol. 16: 1–4.
Baptist, J.N., Gholson, R.K. and Coon, M.J. 1963. Hydrocarbon oxidation by a bacterial enzyme system. I. Products of octane oxidation. Biochim. Biophys. Acta 69: 40–47.
Nunn, W.D. 1986. A molecular view of fatty acid catabolism in E. coli. Microbiol. Rev. 50: 179–192.
Inoue, A. and Horikoshi, K. . 1989. A Pseudomonas thrives in high concentrations of toluene. Nature 338: 264–266.
Rezessy, J.M., Huijberts, G.N.M. and de Bont, J. A. M. 1986. Potential of organic solvents in cultivating micro-organisms on toxic water-insoluble compounds. Laane, C., Tramper, J. and Lilly, M. D. (Eds.). Biocatalysis in Organic Media. Elsevier Science Publishers B. V., Amsterdam.
de Smet, M.J. 1982. Thesis, University of Groningen, The Netherlands.
Van Heerikhuizen, H., Kwak, E., Van Bruggen, E.F.J. and Witholt, B. 1975. Characterization of a low density cytoplasmic membrane subfraction isolated from Escherichia coli. Biochim. Biophys. Acta 413: 177–191.
Lounatmaa, K. and Nanninga, N. 1976. Effect of polymyxin on the outer membrane of Salmonella typhimuriumn freeze-fracture studies. J. Bacteriol. 128: 665–667.
Laane, C., Boeren, S., Vos, K. and Veeger, C. 1987. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 30: 81–87.
Overath, P., Pauli, G. and Schairer, H.U. 1969. Fatty acid degradation in Escherichia coli. Eur. J. Biochem. 7: 559–574.
Eggink, G., Lageveen, R.G., Altenburg, B. and Witholt, B. 1987. Gontroiled and functional expression of P. oleovorans alkane utilizing system in Pseudomonas putida and Escherichia coli. J. Biol. Chem. 262: 17712–17718.
Mason, C.A. and Bailey, J.E. 1989. Effects of plasmid presence on growth and enzyme activity of Escherichia coli DH5 α. Appl. Microbiol. Biotechnol. 32: 54–60.
de Smet, M. J., Kingma, J., Wynberg, H. and Witholt, B. 1983. Pseudomonas oleovorans as a tool in bioconversions of hydrocarbons: growth, morphology and conversion characteristics in different two-phase systems. Enz. Microb. Technol. 5: 352–360.
de Smet, M. J., Kingma, J. and Witholt, B. 1978. The effect of toluene on the structure and permeability of the outer and the cytoplasmic membranes of Escherichia coli. Biochim. Biophys. Acta 506: 64–80.
Witholt, B., de Smet, M.J., Kingma, J., Van Beilen, J., Kok, M., Lageveen, R. G. and Eggink, G. 1990. Bioconversions of aliphatic compounds by Pseudomonas oleovorans in multiphase bioreactors: background and economic potential. TIBTECH 7: 46–52.
Bachman, B.J. 1987. Derivaties and genotypes of some mutant derivatives of Escherichia coli K-12. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology, vol. 2. Neidhardt, F. C., Ingraham, J. L., Low, K. B., Magasanik, B., Schaechter, M., and Umbarger, H. E. (Eds.) American Society for Microbiology, Washington, D.C.
Lageveen, R.G., Huisman, G.W., Preusting, H., Ketelaar, P., Eggink, G. and Witholt, B. 1988. Formation of polyesters by P. oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-hydroxyalkenoates. Appl. Environ. Microbiol. 54: 2924–2932.
Manniatis, T., Fritsch, E.F. and Sambrook, J. (1982) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Lab., Cold Spring Harbor, NY.
Witholt, B. 1972. Method for isolating mutants overproducing nicotinamide adenine dinucleotide and its precursors. J. Bacteriol. 109: 350–364.
Rekker, R.F. and de Kort, H. M. 1979. The hydrophobic fragmental constant. Eur. J. Med. Chem. 14: 479–488.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Favre-Bulle, O., Schouten, T., Kingma, J. et al. Bioconversion of N-Octane to Octanoic Acid by a Recombinant Escherichia Coli Cultured in a Two-Liquid Phase Bioreactor. Nat Biotechnol 9, 367–371 (1991). https://doi.org/10.1038/nbt0491-367
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0491-367
- Springer Nature America, Inc.
This article is cited by
-
Modulating the import of medium-chain alkanes in E. coli through tuned expression of FadL
Journal of Biological Engineering (2016)