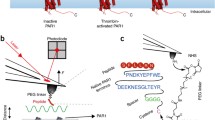
Abstract
Although membrane proteins are ubiquitous within all living organisms and represent the majority of drug targets, a general method for direct, label-free measurement of ligand binding to native membranes has not been reported. Here we show that backscattering interferometry (BSI) can accurately quantify ligand-receptor binding affinities in a variety of membrane environments. By detecting minute changes in the refractive index of a solution, BSI allows binding interactions of proteins with their ligands to be measured at picomolar concentrations. Equilibrium binding constants in the micromolar to picomolar range were obtained for small- and large-molecule interactions in both synthetic and cell-derived membranes without the use of labels or supporting substrates. The simple and low-cost hardware, high sensitivity and label-free nature of BSI should make it readily applicable to the study of many membrane-associated proteins of biochemical and pharmacological interest.


Similar content being viewed by others
References
Krummel, M.F. & Davis, M.M. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr. Opin. Immunol. 14, 66–74 (2002).
Overington, J.P., Al-Lazikani, B. & Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006).
Wise, A., Gearing, K. & Rees, S. Target validation of G-protein coupled receptors. Drug Discov. Today 7, 235–246 (2002).
Markov, D.A., Swinney, K. & Bornhop, D.J. Label-free molecular interaction determinations with nanoscale interferometry. J. Am. Chem. Soc. 126, 16659–16664 (2004).
Sorensen, H.S., Larsen, N.B., Latham, J.C., Bornhop, D.J. & Andersen, P.E. Highly sensitive biosensing based on interference from light scattering in capillary tubes. Appl. Phys. Lett. 89, 151108 (2006).
Bornhop, D.J. et al. Free-solution, label-free molecular interactions studied by back-scattering interferometry. Science 317, 1732–1736 (2007).
Kussrow, A. et al. Measurement of monovalent and polyvalent carbohydrate-lectin binding by back-scattering interferometry. Anal. Chem. 81, 4889–4897 (2009).
Sonnino, S., Mauri, L., Chigorno, V. & Prinetti, A. Gangliosides as components of lipid membrane domains. Glycobiology 17, 1R–13R (2007).
Fishman, P.H., Pacuszka, T. & Orlandi, P.A. Gangliosides as receptors for bacterial enterotoxins. Adv. Lipid Res. 25, 165–187 (1993).
Kuziemko, G.M., Stroh, M. & Stevens, R.C. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry 35, 6375–6384 (1996).
Fang, Y., Frutos, A. & Lahiri, J. Ganglioside microarrays for toxin detection. Langmuir 19, 1500–1505 (2003).
Cannon, B. et al. Cholesterol modulated antibody binding in supported lipid membranes as determined by total internal reflectance microscopy on a microfabricated high-throughput glass chip. Langmuir 21, 9666–9674 (2005).
Brian, A.A. & McConnell, H.M. Allogenic stimulation of cyto-toxic T-cell by supported planar membranes. Proc. Natl. Acad. Sci. USA 81, 6159–6163 (1984).
Mossman, K.D., Campi, G., Groves, J.T. & Dustin, M.L. Altered TCR signaling from geometrically repatterned immunological synapses. Science 310, 1191–1193 (2005).
Cravatt, B.F. & Lichtman, A.H. Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system. Curr. Opin. Chem. Biol. 7, 469–475 (2003).
Devane, W.A. et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992).
Mileni, M. et al. Binding and inactivation mechanism of a humanized fatty acid amide hydrolase by α-ketoheterocycle inhibitors revealed from co-crystal structures. J. Am. Chem. Soc. 131, 10497–10506 (2009).
Boger, D.L. et al. Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. J. Med. Chem. 48, 1849–1856 (2005).
Romero, F.A. et al. Potent and selective alpha-ketoheterocycle-based inhibitors of the anandamide and oleamide catabolizing enzyme, fatty acid amide hydrolase. J. Med. Chem. 50, 1058–1068 (2007).
Garfunkle, J. et al. Optimization of the central heterocycle of alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase. J. Med. Chem. 51, 4392–4403 (2008).
Cheng, Y. & Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 (1973).
Hesselgesser, J. et al. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J. Immunol. 160, 877–883 (1998).
Pin, J.-P. et al. Activation mechanism of the heterodimeric GABAB receptor. Biochem. Pharmacol. 68, 1565–1572 (2004).
Urwyler, S. et al. Positive allosteric modulation of native and recombinant gamma-aminobutyric acidB receptors by 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol. Pharm. 60, 963–971 (2001).
Bowery, N.G., Hill, D.R. & Hudson, A.L. [3Hl(-)baclofen: an improved ligand for GABAB sites. Neuropharmacology 24, 207–210 (1985).
Howson, W., Mistry, J., Broekman, M. & Hills, J.M. Biological activity of 3-aminopropyl (methyl) phosphinic acid, a potent and selective GABAB agonist with CNS activity. Bioorg. Med. Chem. Lett. 3, 515–518 (1993).
Froestl, W. et al. Phosphinic acid analogs of GABA. 1. New potent and selective GABAB agonists. J. Med. Chem. 38, 3297–3312 (1995).
Brugger, F., Wicki, U., Olpe, H.R., Froestl, W. & Mickel, S. The action of new potent GABA-B receptor antagonists in the hemisected spinal cord preparation of the rat. Eur. J. Pharmacol. 235, 153–155 (1993).
Latham, J.C., Stein, R.A., Bornhop, D.J. & Mchaourab, H.S. Free-solution label-free detection of α-crystallin chaperone interactions by back-scattering interferometry. Anal. Chem. 81, 1865–1871 (2009).
Burns, J.M. et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 203, 2201–2213 (2006).
Kaupmann, K. et al. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386, 239–246 (1997).
Kussrow, A.K. et al. The potential of backscattering interferometry as an in vitro clinical diagnostic tool for the serological diagnosis of infectious disease. Analyst (Lond.) 135, 1535–1537 (2010).
Kussrow, A., Baksh, M.M., Bornhop, D.J. & Finn, M.G. Universal sensing by transduction of antibody binding using backscattering interferometry. ChemBioChem 12, 367–370 (2011).
Acknowledgements
This work was supported by the US National Institutes of Health (RO1 EB003537-01A2; U01 MH069062; and the Joint Center for Innovative Membrane Protein Technologies, Roadmap Grant GM073197) and The Skaggs Institute for Chemical Biology. We are grateful to J. Garfunkel and D. Boger of The Scripps Research Institute for samples of the FAAH inhibitors, R. Stevens of The Scripps Research Institute for samples of the FAAH protein, M. Hanes and T. Handel of the University of California, San Diego for the samples of the CXCL12 chemokine and K. Kaupmann of Novartis for the GABAB-transfected CHO cell line.
Author information
Authors and Affiliations
Contributions
M.M.B. developed methods for sample preparation, prepared samples for analysis and processed the raw BSI data; A.K.K. performed BSI measurements and processed the raw data; M.M. prepared the FAAH protein; M.M.B., A.K.K., M.G.F. and D.J.B. designed the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.J.B. has a financial interest in a company that is commercializing BSI. The other authors declare that they have no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figs. 1–3 (PDF 854 kb)
Rights and permissions
About this article
Cite this article
Baksh, M., Kussrow, A., Mileni, M. et al. Label-free quantification of membrane-ligand interactions using backscattering interferometry. Nat Biotechnol 29, 357–360 (2011). https://doi.org/10.1038/nbt.1790
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1790
- Springer Nature America, Inc.
This article is cited by
-
Electric field-enhanced backscatter interferometry detection for capillary electrophoresis
Scientific Reports (2024)
-
Label-free quantification of calcium-sensor targeting to photoreceptor guanylate cyclase and rhodopsin kinase by backscattering interferometry
Scientific Reports (2017)
-
Biophysics in drug discovery: impact, challenges and opportunities
Nature Reviews Drug Discovery (2016)
-
Quantification of Plasmodium-host protein interactions on intact, unmodified erythrocytes by back-scattering interferometry
Malaria Journal (2015)
-
Label-free detection and identification of protein ligands captured by receptors in a polymerized planar lipid bilayer using MALDI-TOF MS
Analytical and Bioanalytical Chemistry (2015)