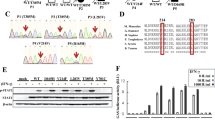
Abstract
The magnesium ion, Mg2+, is essential for all life as a cofactor for ATP, polyphosphates such as DNA and RNA, and metabolic enzymes, but whether it plays a part in intracellular signalling (as Ca2+ does) is unknown. Here we identify mutations in the magnesium transporter gene, MAGT1, in a novel X-linked human immunodeficiency characterized by CD4 lymphopenia, severe chronic viral infections, and defective T-lymphocyte activation. We demonstrate that a rapid transient Mg2+ influx is induced by antigen receptor stimulation in normal T cells and by growth factor stimulation in non-lymphoid cells. MAGT1 deficiency abrogates the Mg2+ influx, leading to impaired responses to antigen receptor engagement, including defective activation of phospholipase Cγ1 and a markedly impaired Ca2+ influx in T cells but not B cells. These observations reveal a role for Mg2+ as an intracellular second messenger coupling cell-surface receptor activation to intracellular effectors and identify MAGT1 as a possible target for novel therapeutics.






Similar content being viewed by others
Accession codes
Data deposits
The Illumina sequencing data has been deposited in dbGaP with accession code phs000365.v1.p1.
References
Cakmak, I. & Kirkby, E. A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Planta 133, 692–704 (2008)
Cowan, J. A. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals 15, 225–235 (2002)
Yang, W., Lee, J. Y. & Nowotny, M. Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol. Cell 22, 5–13 (2006)
Gasser, A., Bruhn, S. & Guse, A. H. Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J. Biol. Chem. 281, 16906–16913 (2006)
Grubbs, R. D. & Maguire, M. E. Magnesium as a regulatory cation: criteria and evaluation. Magnesium 6, 113–127 (1987)
Murphy, E. Mysteries of magnesium homeostasis. Circ. Res. 86, 245–248 (2000)
Permyakov, E. A. & Kretsinger, R. H. Cell signaling, beyond cytosolic calcium in eukaryotes. J. Inorg. Biochem. 103, 77–86 (2009)
Takaya, J., Higashino, H. & Kobayashi, Y. Can magnesium act as a second messenger? Current data on translocation induced by various biologically active substances. Magnes. Res. 13, 139–146 (2000)
Hogan, P. G., Lewis, R. S. & Rao, A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 28, 491–533 (2010)
Romani, A. M. Magnesium homeostasis in mammalian cells. Front. Biosci. 12, 308–331 (2007)
Abboud, C. N., Scully, S. P., Lichtman, A. H., Brennan, J. K. & Segel, G. B. The requirements for ionized calcium and magnesium in lymphocyte proliferation. J. Cell. Physiol. 122, 64–72 (1985)
Modiano, J. F., Kelepouris, E., Kern, J. A. & Nowell, P. C. Requirement for extracellular calcium or magnesium in mitogen-induced activation of human peripheral blood lymphocytes. J. Cell. Physiol. 135, 451–458 (1988)
Whitney, R. B. & Sutherland, R. M. The influence of calcium, magnesium and cyclic adenosine 3′,5′-monophosphate on the mixed lymphocyte reaction. J. Immunol. 108, 1179–1183 (1972)
Ng, L. L., Davies, J. E. & Garrido, M. C. Intracellular free magnesium in human lymphocytes and the response to lectins. Clin. Sci. (Lond.) 80, 539–547 (1991)
Rijkers, G. T. & Griffioen, A. W. Changes in free cytoplasmic magnesium following activation of human lymphocytes. Biochem. J. 289, 373–377 (1993)
Chun, H. J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002)
Notarangelo, L. D. Primary immunodeficiencies. J. Allergy Clin. Immunol. 125, S182–S194 (2010)
Zhang, Q. et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361, 2046–2055 (2009)
Chan, A. C. et al. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science 264, 1599–1601 (1994)
Peterson, E. J. & Koretzky, G. A. Signal transduction in T lymphocytes. Clin. Exp. Rheumatol. 17, 107–114 (1999)
Arpaia, E., Shahar, M., Dadi, H., Cohen, A. & Roifman, C. M. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell 76, 947–958 (1994)
Feske, S. et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 (2006)
Unexplained CD4+ T-lymphocyte depletion in persons without evident HIV infection — United States. MMWR Morb. Mortal. Wkly. Rep. 41, 541–545 (1992)
Laurence, J., Siegal, F. P., Schattner, E., Gelman, I. H. & Morse, S. Acquired immunodeficiency without evidence of infection with human immunodeficiency virus types 1 and 2. Lancet 340, 273–274 (1992)
Smith, D. K., Neal, J. J. & Holmberg, S. D. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. An investigation of cases in the United States. N. Engl. J. Med. 328, 373–379 (1993)
Fauci, A. S. CD4+ T-lymphocytopenia without HIV infection — no lights, no camera, just facts. N. Engl. J. Med. 328, 429–431 (1993)
Freier, S. et al. Hereditary CD4+ T lymphocytopenia. Arch. Dis. Child. 78, 371–372 (1998)
Lin, S. J., Chao, H. C., Yan, D. C. & Kuo, M. L. Idiopathic CD4+ T lymphocytopenia in two siblings. Pediatr. Hematol. Oncol. 18, 153–156 (2001)
Lobato, M. N., Spira, T. J. & Rogers, M. F. CD4+ T lymphocytopenia in children: lack of evidence for a new acquired immunodeficiency syndrome agent. Pediatr. Infect. Dis. J. 14, 527–535 (1995)
Junge, S. et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur. J. Immunol. 37, 3270–3280 (2007)
Kimmig, S. et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J. Exp. Med. 195, 789–794 (2002)
Kohler, S. & Thiel, A. Life after the thymus: CD31+ and CD31− human naive CD4+ T-cell subsets. Blood 113, 769–774 (2009)
Wengler, G. S. et al. A PCR-based non-radioactive X-chromosome inactivation assay for genetic counseling in X-linked primary immunodeficiencies. Life Sci. 61, 1405–1411 (1997)
Goytain, A. & Quamme, G. A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics 6, 48–66 (2005)
Quamme, G. A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 298, C407–C429 (2010)
Zhou, H. & Clapham, D. E. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc. Natl Acad. Sci. USA 106, 15750–15755 (2009)
Xie, Z., Peng, J., Pennypacker, S. D. & Chen, Y. Critical role for the catalytic activity of phospholipase C-γ1 in epidermal growth factor-induced cell migration. Biochem. Biophys. Res. Commun. 399, 425–428 (2010)
Weiss, A. & Littman, D. R. Signal transduction by lymphocyte antigen receptors. Cell 76, 263–274 (1994)
Nel, A. E. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J. Allergy Clin. Immunol. 109, 758–770 (2002)
Sutherland, E. W. Studies on the mechanism of hormone action. Science 177, 401–408 (1972)
Flynn, A. Control of in vitro lymphocyte proliferation by copper, magnesium and zinc deficiency. J. Nutr. 114, 2034–2042 (1984)
Sabbagh, F., Lecerf, F., Hulin, A., Bac, P. & German-Fattal, M. Effect of hypomagnesemia on allogeneic activation in mice. Transpl. Immunol. 20, 83–87 (2008)
Jin, J. et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760 (2008)
Cossu, F. Genetics of SCID. Ital. J. Pediatr. 36, 36–76 (2010)
Filipovich, A. H., Zhang, K., Snow, A. L. & Marsh, R. A. X-linked lymphoproliferative syndromes: brothers or distant cousins? Blood 116, 3398–3408 (2010)
Fu, G. et al. Phospholipase Cγ1 is essential for T cell development, activation, and tolerance. J. Exp. Med. 207, 309–318 (2010)
Crabtree, G. R. Contingent genetic regulatory events in T lymphocyte activation. Science 243, 355–361 (1989)
Petrovas, C. et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203, 2281–2292 (2006)
Allen, R. C., Zoghbi, H. Y., Moseley, A. B., Rosenblatt, H. M. & Belmont, J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am. J. Hum. Genet. 51, 1229–1239 (1992)
Weber, K., Bartsch, U., Stocking, C. & Fehse, B. A multicolor panel of novel lentiviral “Gene Ontology” (LeGO) vectors for functional gene analysis. Mol. Ther. 16, 698–706 (2008)
Killilea, D. W. & Ames, B. N. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc. Natl Acad. Sci. USA 105, 5768–5773 (2008)
Acknowledgements
We thank L. Zheng, A. Weiss, R. Germain, R. Siegel, F. Wolf and P. Schwartzberg for critically reading the manuscript; F. Wolf for advice on magnesium assessments; L. Zheng, C. Lowell and A. Weiss for advice on experiments and data; H. Jing for making HVS lines from patient cells; P. Chen for assistance with plasmid DNA preparation; N. Sandler for flow cytometry assistance; A. Snow and H. Jing for assistance with genomic DNA library preparation for Solexa sequencing; J. Almenara and Illumina staff for Solexa assistance; D. Killilea for assistance with MS-ICP data interpretation; and A. Irani for referring the patients. F.-Y.L. is in the Medical Scientist Training Program at the University of California–San Francisco and thanks K. Shannon and J. Toutolmin for support and encouragement. This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
F.-Y.L. characterized the MAGT1 mutations and the TCR activation defect in the patients. B.C.-D. characterized the Mg2+ influx and the signalling defects. B.C.-D., F.-Y.L., H.C.S. and M.J.L. conceived and planned the experiments, and prepared the manuscript. J.C.D. performed the lyonization assay. C.K. performed the RT–PCR experiments. G.U., J.I.C. and H.C.S. referred patients and provided clinical data. H.F.M. coordinated clinical protocol and sample collection. D.C.D. provided assistance with sequencing and flow cytometry, and guided some patient assessments. All authors discussed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1, Supplementary Figures 1-11 with legends, a Supplementary Discussion and additional references. (PDF 1726 kb)
Rights and permissions
About this article
Cite this article
Li, FY., Chaigne-Delalande, B., Kanellopoulou, C. et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 475, 471–476 (2011). https://doi.org/10.1038/nature10246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10246
- Springer Nature Limited
This article is cited by
-
Epstein–Barr virus-associated B-cell lymphoproliferative disorder meeting the definition of CAEBV B cell disease: a case report
BMC Infectious Diseases (2023)
-
A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer
Journal of Health, Population and Nutrition (2023)
-
A RIPK3-independent role of MLKL in suppressing parthanatos promotes immune evasion in hepatocellular carcinoma
Cell Discovery (2023)
-
MAGT1 Gene Mutation is Associated with Myositis and CD127 Expression Downregulation
Journal of Clinical Immunology (2023)
-
Identification of a novel MAGT1 mutation supports a diagnosis of XMEN disease
Genes & Immunity (2022)