Abstract
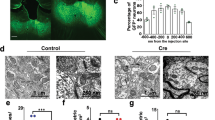
Abnormal activity in the medial prefrontal cortex (mPFC) is consistently observed in neuropsychiatric disorders, but the mechanisms involved remain unclear. Chronic aberrant excitation and/or inhibition of mPFC neurons were proposed to cause cognitive impairments. However, direct evidence for this hypothesis is lacking because it is technically challenging to control synaptic properties in a chronic and locally restricted, yet specific, manner. Here, we generated conditional knockout (cKO) mice of neuroligin-2 (Nlgn2), a postsynaptic cell-adhesion molecule of inhibitory synapses linked to neuropsychiatric disorders. cKO of Nlgn2 in adult mPFC rendered Nlgn2 protein undetectable after already 2–3 weeks, but induced major reductions in synaptic inhibition after only 6–7 weeks, and caused parallel impairments in anxiety, fear memory and social interaction behaviors. Moreover, cKO of Nlgn2 severely impaired behavioral stimulation of immediate-early gene expression in the mPFC, suggesting that chronic reduction in synaptic inhibition uncoupled the mPFC from experience-dependent inputs. Our results indicate that Nlgn2 is required for continuous maintenance of inhibitory synapses in the adult mPFC, and that chronic impairment of local inhibition disengages the mPFC from its cognitive functions by partially uncoupling the mPFC from experience-induced inputs.





Similar content being viewed by others
References
Wise SP . Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 2008; 31: 599–608.
Dalley JW, Cardinal RN, Robbins TW . Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 2004; 28: 771–784.
Amaral DG, Schumann CM, Nordahl CW . Neuroanatomy of autism. Trends Neurosci 2008; 31: 137–145.
Thermenos HW, Keshavan MS, Juelich RJ, Molokotos E, Whitfield-Gabrieli S, Brent BK et al. A review of neuroimaging studies of young relatives of individuals with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2013; 162B: 604–635.
Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G et al. Response to learned threat: An FMRI study in adolescent and adult anxiety. Am J Psychiatry 2013; 170: 1195–1204.
Drevets WC . Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 2000; 126: 413–431.
Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S . Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain 2008; 131: 2479–2488.
Dichter GS, Felder JN, Bodfish JW . Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc Cogn Affect Neurosci 2009; 4: 215–226.
Liu TY, Hsieh JC, Chen YS, Tu PC, Su TP, Chen LF . Different patterns of abnormal gamma oscillatory activity in unipolar and bipolar disorder patients during an implicit emotion task. Neuropsychologia 2012; 50: 1514–1520.
Sokhadze E, Baruth J, Tasman A, Mansoor M, Ramaswamy R, Sears L et al. Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Appl Psychophysiol Biofeedback 2010; 35: 147–161.
Lisman J . Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol 2012; 22: 537–544.
Koike S, Takizawa R, Nishimura Y, Kinou M, Kawasaki S, Kasai K . Reduced but broader prefrontal activity in patients with schizophrenia during n-back working memory tasks: a multi-channel near-infrared spectroscopy study. J Psychiatr Res 2013; 47: 1240–1246.
Etherton MR, Tabuchi K, Sharma M, Ko J, Sudhof TC . An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J 2011; 30: 2908–2919.
Etherton MR, Blaiss CA, Powell CM, Sudhof TC . Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA 2009; 106: 17998–18003.
Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012; 486: 256–260.
Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB . Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 2005; 102: 12560–12565.
Chao HT, Zoghbi HY, Rosenmund C . MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 2007; 56: 58–65.
Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB et al. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature 2012; 489: 385–390.
Mao R, Page DT, Merzlyak I, Kim C, Tecott LH, Janak PH et al. Reduced conditioned fear response in mice that lack Dlx1 and show subtype-specific loss of interneurons. J Neurodev Disord 2009; 1: 224–236.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011; 477: 171–178.
Ichtchenko K, Nguyen T, Sudhof TC . Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem 1996; 271: 2676–2682.
Varoqueaux F, Jamain S, Brose N . Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol 2004; 83: 449–456.
Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM . Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 2004; 119: 1013–1026.
Sun C, Cheng MC, Qin R, Liao DL, Chen TT, Koong FJ et al. Identification and functional characterization of rare mutations of the neuroligin-2 gene (NLGN2) associated with schizophrenia. Hum Mol Genet 2011; 20: 3042–3051.
Roberts JL, Hovanes K, Dasouki M, Manzardo AM, Butler MG . Chromosomal microarray analysis of consecutive individuals with autism spectrum disorders or learning disability presenting for genetic services. Gene 2014; 535: 70–78.
Sudhof TC . Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008; 455: 903–911.
Lee K, Kim Y, Lee SJ, Qiang Y, Lee D, Lee HW et al. MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proc Natl Acad Sci USA 2013; 110: 336–341.
Pettem KL, Yokomaku D, Takahashi H, Ge Y, Craig AM . Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. J Cell Biol 2013; 200: 321–336.
Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 2009; 63: 628–642.
Lionel AC, Vaags AK, Sato D, Gazzellone MJ, Mitchell EB, Chen HY et al. Rare exonic deletions implicate the synaptic organizer Gephyrin (GPHN) in risk for autism, schizophrenia and seizures. Hum Mol Genet 2013; 22: 2055–2066.
Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K et al. Neuroligins determine synapse maturation and function. Neuron 2006; 51: 741–754.
Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 2007; 54: 919–931.
Gibson JR, Huber KM, Sudhof TC . Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. J Neurosci 2009; 29: 13883–13897.
Foldy C, Malenka RC, Sudhof TC . Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron 2013; 78: 498–509.
Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Sudhof TC . Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 2013; 154: 75–88.
Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011; 471: 358–362.
Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Sudhof TC . Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron 2012; 73: 990–1001.
Winslow JT . Mouse social recognition and preference. Curr Protoc Neurosci 2003; Chapter 8: Unit 8 16.
Semyanov A, Walker MC, Kullmann DM, Silver RA . Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci 2004; 27: 262–269.
Dalley JW, Mar AC, Economidou D, Robbins TW . Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 2008; 90: 250–260.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 2004; 3: 287–302.
Morgan JI, Cohen DR, Hempstead JL, Curran T . Mapping patterns of c-fos expression in the central nervous system after seizure. Science 1987; 237: 192–197.
Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C et al. Generation of a synthetic memory trace. Science 2012; 335: 1513–1516.
Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL et al. Creating a false memory in the hippocampus. Science 2013; 341: 387–391.
Xu W, Sudhof TC . A neural circuit for memory specificity and generalization. Science 2013; 339: 1290–1295.
Fonio E, Golani I, Benjamini Y . Measuring behavior of animal models: faults and remedies. Nat Methods 2012; 9: 1167–1170.
Walf AA, Frye CA . The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007; 2: 322–328.
Lee YA, Goto Y . Neurodevelopmental disruption of cortico-striatal function caused by degeneration of habenula neurons. PLoS One 2011; 6: e19450.
Salmond CH, Vargha-Khadem F, Gadian DG, de Haan M, Baldeweg T . Heterogeneity in the patterns of neural abnormality in autistic spectrum disorders: evidence from ERP and MRI. Cortex 2007; 43: 686–699.
Carter EJ, Williams DL, Minshew NJ, Lehman JF . Is he being bad? Social and language brain networks during social judgment in children with autism. PLoS One 2012; 7: e47241.
Parellada M, Penzol MJ, Pina L, Moreno C, Gonzalez-Vioque E, Zalsman G et al. The neurobiology of autism spectrum disorders. Eur Psychiatry 2014; 29: 11–19.
Brune M, Lissek S, Fuchs N, Witthaus H, Peters S, Nicolas V et al. An fMRI study of theory of mind in schizophrenic patients with "passivity" symptoms. Neuropsychologia 2008; 46: 1992–2001.
Bosia M, Riccaboni R, Poletti S . Neurofunctional correlates of theory of mind deficits in schizophrenia. Curr Top Med Chem 2012; 12: 2284–2302.
Hoover WB, Vertes RP . Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 2007; 212: 149–179.
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ . Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 2005; 492: 145–177.
Lee AT, Gee SM, Vogt D, Patel T, Rubenstein JL, Sohal VS . Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron 2014; 81: 61–68.
Koistinaho J, Hicks KJ, Sagar SM . Tetrodotoxin enhances light-induced c-fos gene expression in the rabbit retina. Brain Res Mol Brain Res 1993; 17: 179–183.
Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S . AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci 2006; 9: 887–895.
Sheng M, Greenberg ME . The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 1990; 4: 477–485.
Perez-Cadahia B, Drobic B, Davie JR . Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol 2011; 89: 61–73.
Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF . Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA 2011; 108: 2587–2592.
Acknowledgements
We thank Stephan Maxeiner and Bo Zhang for advice. This study was supported by grants from the NIH (MH086403 to LC and TCS; MH091193 to LC and 1K99MH09915301 to WX) and the Simons Foundation (307762 to TCS). JL was supported by National Natural Science Foundation of China (31271098).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Liang, J., Xu, W., Hsu, YT. et al. Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol Psychiatry 20, 850–859 (2015). https://doi.org/10.1038/mp.2015.31
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.31
- Springer Nature Limited
This article is cited by
-
The developmental timing of spinal touch processing alterations predicts behavioral changes in genetic mouse models of autism spectrum disorders
Nature Neuroscience (2024)
-
Schizophrenia-associated LRRTM1 regulates cognitive behavior through controlling synaptic function in the mediodorsal thalamus
Molecular Psychiatry (2021)
-
Medial prefrontal cortex (A32 and A25) projections in the common marmoset: a subcortical anterograde study
Scientific Reports (2021)
-
Neuroligin 2 regulates absence seizures and behavioral arrests through GABAergic transmission within the thalamocortical circuitry
Nature Communications (2020)
-
Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex
Nature Communications (2020)