Abstract
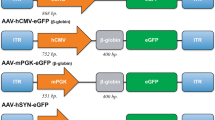
Adeno-associated viral (AAV) vectors based on serotype 5 are an efficient means to target dorsal root ganglia (DRG) to study gene function in the primary sensory neurons of the peripheral nervous system. In this study, we have developed a compact AAV dual promoter vector composed of the cytomegalovirus (CMV) and chicken beta-actin (CAG) promoters in a back-to-back configuration with a shared enhancer, and show efficient expression of two proteins simultaneously in DRG neurons. We demonstrate how this is useful for experiments on axonal regeneration, by co-expressing a gene of interest and an axonal marker. Using a farnesylated form of eGFP, which is actively transported along axons, we show superior long-distance labelling of axons of DRG neurons compared with normal eGFP. Additionally, we have efficiently transduced lumbar DRG neurons by injecting the AAV dual promoter vector into the dorsal intrathecal space, which is a less invasive delivery method. In summary, we have developed an AAV dual promoter vector designed for simultaneous expression of a gene of interest and a fluorescent protein to label long-distance axonal projections, which allows specific quantification of axons from transduced neurons after injury.





Similar content being viewed by others
References
Mason MR, Tannemaat MR, Malessy MJ, Verhaagen J . Gene therapy for the peripheral nervous system: a strategy to repair the injured nerve? Curr Gene Ther 2011; 11: 75–89.
Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M et al. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc Natl Acad Sci USA 2011; 108: E99–E107.
Hoyng SA, Tannemaat MR, De WF, Verhaagen J, Malessy MJ . Nerve surgery and gene therapy: a neurobiological and clinical perspective. J Hand Surg Eur Vol 2011; 36: 735–746.
Beutler AS, Banck MS, Walsh CE, Milligan ED . Intrathecal gene transfer by adeno-associated virus for pain. Curr Opin Mol Ther 2005; 7: 431–439.
Beutler AS, Reinhardt M . AAV for pain: steps towards clinical translation. Gene Therapy 2009; 16: 461–469.
Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B et al. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci 2009; 29: 5546–5557.
Tan CL, Andrews MR, Kwok JC, Heintz TG, Gumy LF, Fassler R et al. Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J Neurosci 2012; 32: 7325–7335.
Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G et al. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp-mediated effects. Exp Neurol 2012; 235: 162–173.
Hou S, Nicholson L, van NE, Motsch M, Blesch A . Dependence of regenerated sensory axons on continuous neurotrophin-3 delivery. J Neurosci 2012; 32: 13206–13220.
Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron 2009; 64: 165–172.
Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 2007; 369: 2097–2105.
Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet 1994; 8: 148–154.
Storek B, Reinhardt M, Wang C, Janssen WG, Harder NM, Banck MS et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci USA 2008; 105: 1055–1060.
Storek B, Harder NM, Banck MS, Wang C, McCarty DM, Janssen WG et al. Intrathecal long-term gene expression by self-complementary adeno-associated virus type 1 suitable for chronic pain studies in rats. Mol Pain 2006; 2: 4.
Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I . Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain 2009; 5: 52.
Vulchanova L, Schuster DJ, Belur LR, Riedl MS, Podetz-Pedersen KM, Kitto KF et al. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain 2010; 6: 31.
Xu Q, Chou B, Fitzsimmons B, Miyanohara A, Shubayev V, Santucci C et al. In vivo gene knockdown in rat dorsal root ganglia mediated by self-complementary adeno-associated virus serotype 5 following intrathecal delivery. PLoS One 2012; 7: e32581.
Mason MR, Ehlert EM, Eggers R, Pool CW, Hermening S, Huseinovic A et al. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther 2010; 18: 715–724.
Aronheim A . Ras signaling pathway for analysis of protein-protein interactions in yeast and mammalian cells. Methods Mol Biol 2004; 250: 251–262.
Hancock JF, Cadwallader K, Paterson H, Marshall CJA . CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J 1991; 10: 4033–4039.
Jiang W, Hunter T . Analysis of cell-cycle profiles in transfected cells using a membrane-targeted GFP. Biotechniques 1998; 24: 349–350; 352, 354.
Martinez-Salas E . Internal ribosome entry site biology and its use in expression vectors. Curr Opin Biotechnol 1999; 10: 458–464.
Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T . IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther 2000; 1: 376–382.
Sokolic RA, Sekhsaria S, Sugimoto Y, Whiting-Theobald N, Linton GF, Li F et al. A bicistronic retrovirus vector containing a picornavirus internal ribosome entry site allows for correction of X-linked CGD by selection for MDR1 expression. Blood 1996; 87: 42–50.
Hennecke M, Kwissa M, Metzger K, Oumard A, Kroger A, Schirmbeck R et al. Composition and arrangement of genes define the strength of IRES-driven translation in bicistronic mRNAs. Nucleic Acids Res 2001; 29: 3327–3334.
Kozak M . Alternative ways to think about mRNA sequences and proteins that appear to promote internal initiation of translation. Gene 2003; 318: 1–23.
Bochkov YA, Palmenberg AC . Translational efficiency of EMCV IRES in bicistronic vectors is dependent upon IRES sequence and gene location. Biotechniques 2006; 41: 283–284; 286, 288.
Low K, Blesch A, Herrmann J, Tuszynski MH . A dual promoter lentiviral vector for the in vivo evaluation of gene therapeutic approaches to axon regeneration after spinal cord injury. Gene Therapy 2010; 17: 577–591.
Donnelly ML, Luke G, Mehrotra A, Li X, Hughes LE, Gani D et al. Analysis of the aphthovirus 2A/2B polyprotein 'cleavage' mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal 'skip'. J Gen Virol 2001; 82: 1013–1025.
Ryan MD, King AM, Thomas GP . Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol 1991; 72 (Pt 11): 2727–2732.
Ryan MD, Drew J . Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J 1994; 13: 928–933.
Szymczak AL, Vignali DA . Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther 2005; 5: 627–638.
Xu R, Janson CG, Mastakov M, Lawlor P, Young D, Mouravlev A et al. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Therapy 2001; 8: 1323–1332.
Shevtsova Z, Malik JM, Michel U, Bahr M, Kugler S . Promoters and serotypes: targeting of adeno-associated virus vectors for gene transfer in the rat central nervous system in vitro and in vivo. Exp Physiol 2005; 90: 53–59.
Fitzsimons HL, Bland RJ, During MJ . Promoters and regulatory elements that improve adeno-associated virus transgene expression in the brain. Methods 2002; 28: 227–236.
Liu Y, Okada T, Nomoto T, Ke X, Kume A, Ozawa K et al. Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp Mol Med 2007; 39: 170–175.
Niwa H, Yamamura K, Miyazaki J . Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991; 108: 193–199.
Ohlfest JR, Frandsen JL, Fritz S, Lobitz PD, Perkinson SG, Clark KJ et al. Phenotypic correction and long-term expression of factor VIII in hemophilic mice by immunotolerization and nonviral gene transfer using the Sleeping Beauty transposon system. Blood 2005; 105: 2691–2698.
Malkmus SA, Yaksh TL . Intrathecal catheterization and drug delivery in the rat. Methods Mol Med 2004; 99: 109–121.
Yaksh TL, Rudy TA . Chronic catheterization of the spinal subarachnoid space. Physiol Behav 1976; 17: 1031–1036.
Barrett LW, Fletcher S, Wilton SD . Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell Mol Life Sci 2012; 69: 3613–3634.
Paterna JC, Moccetti T, Mura A, Feldon J, Bueler H . Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Therapy 2000; 7: 1304–1311.
Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ . Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther 1999; 10: 2295–2305.
Zufferey R, Donello JE, Trono D, Hope TJ . Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol 1999; 73: 2886–2892.
Cai D, Cohen KB, Luo T, Lichtman JW, Sanes JR . Improved tools for the Brainbow toolbox. Nat Methods 2013; 10: 540–547.
Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 2007; 450: 56–62.
Fleming J, Ginn SL, Weinberger RP, Trahair TN, Smythe JA, Alexander IE . Adeno-associated virus and lentivirus vectors mediate efficient and sustained transduction of cultured mouse and human dorsal root ganglia sensory neurons. Hum Gene Ther 2001; 12: 77–86.
Glatzel M, Flechsig E, Navarro B, Klein MA, Paterna JC, Bueler H et al. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci USA 2000; 97: 442–447.
Harrison PT, Dalziel RG, Ditchfield NA, Quinn JP . Neuronal-specific and nerve growth factor-inducible expression directed by the preprotachykinin-A promoter delivered by an adeno-associated virus vector. Neuroscience 1999; 94: 997–1003.
McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ . Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Therapy 2003; 10: 2112–2118.
Ahmed BY, Chakravarthy S, Eggers R, Hermens WT, Zhang JY, Niclou SP et al. Efficient delivery of Cre-recombinase to neurons in vivo and stable transduction of neurons using adeno-associated and lentiviral vectors. BMC Neurosci 2004; 5: 4.
Ruitenberg MJ, Eggers R, Boer GJ, Verhaagen J . Adeno-associated viral vectors as agents for gene delivery: application in disorders and trauma of the central nervous system. Methods 2002; 28: 182–194.
Acknowledgements
This study was partially funded by grants from the International Spinal Reseach Trust (Strategy Grant STR111) and the Netherlands Organisation for Scientific Research (NWO-grant 40-00812-98-110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was carried out in Amsterdam, The Netherlands.
Rights and permissions
About this article
Cite this article
Fagoe, N., Eggers, R., Verhaagen, J. et al. A compact dual promoter adeno-associated viral vector for efficient delivery of two genes to dorsal root ganglion neurons. Gene Ther 21, 242–252 (2014). https://doi.org/10.1038/gt.2013.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.71
- Springer Nature Limited
Keywords
This article is cited by
-
Improving adeno-associated viral (AAV) vector-mediated transgene expression in retinal ganglion cells: comparison of five promoters
Gene Therapy (2023)
-
Optimization of adeno-associated viral vector-mediated transduction of the corticospinal tract: comparison of four promoters
Gene Therapy (2021)
-
Generation and validation of novel adeno-associated viral vectors for the analysis of Ca2+ homeostasis in motor neurons
Scientific Reports (2017)