Abstract
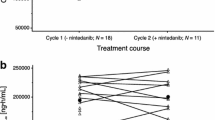
Raltitrexed, a thymidylate synthase inhibitor, was given to 21 patients with advanced small-cell lung cancer, at a dose of 3 mg m(-2) as a 15-min intravenous infusion at 21-day intervals. All of the patients had extensive disease and 17 had received prior therapy. Patients with disease refractory to primary chemotherapy were excluded. Forty-one treatment cycles were given (median two, range one to four). The drug was well tolerated. No objective tumour response was documented. The patients had chemoresistant disease, as shown by a response in only one of ten patients who went on to receive alternative cytotoxic regimens. We conclude that raltitrexed given in this schedule is inactive as second line therapy for small-cell lung cancer.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Woll, P., Basser, R., Le Chevalier, T. et al. Phase II trial of raltitrexed ('Tomudex') in advanced small-cell lung cancer. Br J Cancer 76, 264–265 (1997). https://doi.org/10.1038/bjc.1997.373
Issue Date:
DOI: https://doi.org/10.1038/bjc.1997.373
- Springer Nature Limited
This article is cited by
-
From methotrexate to pemetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates
Investigational New Drugs (2006)