Abstract
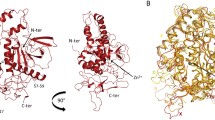
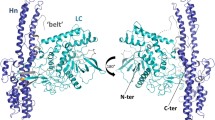
Botulinum neurotoxin serotype B is a zinc protease that disrupts neurotransmitter release by cleaving synaptobrevin-II (Sb2), one of three SNARE proteins involved in neuronal synaptic vesicle fusion. The three-dimensional crystal structure of the apo botulinum neurotoxin serotype B catalytic domain (BoNT/B-LC) has been determined to 2.2 Å resolution, and the complex of cleaved Sb2 with the catalytic domain (Sb2–BoNT/B-LC) has been determined to 2.0 Å resolution. A comparison of the holotoxin catalytic domain and the isolated BoNT/B-LC structure shows a rearrangement of three active site loops. This rearrangement exposes the BoNT/B active site. The Sb2–BoNT/B-LC structure illustrates two distinct binding regions, which explains the specificity of each botulinum neurotoxin for its synaptic vesicle protein. This observation provides an explanation for the proposed cooperativity between binding of full-length substrate and catalysis and suggest a mechanism of synaptobrevin proteolysis employed by the clostridial neurotoxins.





Similar content being viewed by others
References
Simpson, L.L. Botulinum neurotoxin and tetanus toxin. Academic Press, San Diego (1989).
Blasi, J. et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365, 160– 163 (1993).
Schiavo, G., Rossetto, O., Santucci, A., DasGupta, B.R. & Montecucco, C. Botulinum neurotoxins are zinc proteins. J. Biol. Chem. 267, 23479– 23483 (1992).
Montecucco, C. & Schiavo, G. Structure and function of tetanus and botulinum neurotoxins. Q. Rev. Biophys. 28, 423– 472 (1995).
Krieglstein, K.G., DasGupta, B.R. & Henschen, A.H. Covalent structure of botulinum neurotoxin type A: location of sulfhydryl groups, and disulfide bridges and identification of C-termini of light and heavy chains. J. Prot. Chem. 13, 49– 57 (1994).
Simpson, L.L. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J. Pharmacol. Exp. Ther. 212, 16– 21 (1980).
Boquet, P., Duflot, E. & Hauttecoeur, B. Low pH induces a hydrophobic domain in the tetanus toxin molecule. Eur. J. Biochem. 144, 339– 344 (1984).
Lacy, D.B., Stevens, R.C. Sequence homology and structural analysis of the clostridial neurotoxins. J. Mol. Biol. 291, 1091– 1104 (1999).
Boquet, P. & Duflot, E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc. Natl. Acad. Sci. USA 79, 7614– 7618 (1982).
Sudhof, T. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature 375, 645– 653 (1995).
Hanson, P.I., Heuser, J.E. & Jahn, R. Neurotransmitter release — four years of SNARE complexes. Curr. Opin. Neurobiol. 7, 310– 315 (1997).
Sudhof, T.C., De Camilli, P., Niemann, H. & Jahn, R. Membrane fusion machinery: insights from synaptic proteins. Cell 75, 1– 4 (1993).
Swaminathan, S. & Eswaramoorthy, S. The catalytic and binding sites of Clostridium botulinum neurotoxin B defined by structural analysis. Nature Struct. Biol. 3, 693– 699 (2000).
Kurazono, H. et al. Minimal essential domains specifying toxicity of the light chains of tetanus toxin and botulinum neurotoxin type A. J. Biol. Chem. 267, 14721– 14729 (1992).
Lebeda, F.J. & Olson, M.A. Secondary structural predictions for the clostridial neurotoxins. Proteins 20, 293– 300 (1994).
Lacy, D.B., Tepp, W., Cohen, A.C., DasGupta, B.R. & Stevens, R.C. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nature Struct. Biol. 5, 898– 902 (1998).
Pellizzari, R., et al. Structural determinants of the specificity for synaptic vesicle-associated membrane protein/synaptobrevin of tetanus and botulinum type B and G neurotoxins. J. Biol. Chem. 271, 20353– 20358 (1996).
Shone, C.C., et al. Proteolytic cleavage of synthetic fragments of vesicle-associated membrane protein, isoform-2 by botulinum type B neurotoxin. Eur. J. Biochem. 217, 965– 971 (1993).
Yamasaki, S. et al. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J. Biol. Chem. 269, 12764– 12772 (1994).
Binz, T. et al. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J. Biol. Chem. 269, 1617– 1620 (1994).
Cornille, F. et al. Cooperative exosite-dependent cleavage of synaptobrevin by tetanus toxin light chain. J. Biol. Chem. 272, 3459– 3464 (1997).
Hazzard, J., Sudhof, T.C. & Rizo, J. NMR analysis of the structure of synaptobrevin and of its interaction with syntaxin. J. Biomol. NMR. 14, 203– 207 (1999).
Fasshauer, D., Sutton, R.B., Brünger, A.T. & Jahn, R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA 95, 15781– 15786 (1998).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brünger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347– 353 (1998).
Matthews, B.W. Structural basis of the action of thermolysin and related zinc peptidases. Acc. Chem. Res. 21, 333– 340 (1988).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307– 326 (1997).
Navaza, J. AMoRe and automated package for molecular replacement. Acta Crystallogr. A 50, 157– 163 (1994).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110– 119 (1991).
Collaborative Computational Project Number 4. CCP4 Suite: programs for crystallography. Acta Crystallogr. D 50, 760– 763 (1994).
Brünger, A.T. et al. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905– 921 (1998).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946– 950 (1991).
Merritt, E.A. & Bacon, J.A. Raster3D: photorealistic molecular graphic. Methods Enzymol. 277, 505– 524 (1997).
Esnouf, R.M. Further additions to MolScript version 1.4, including reading and contouring of electron-density maps. Acta. Crystallogr. D 55, 938– 940 (1999).
Acknowledgements
We dedicate this paper to H. Niemann, who provided the original light chain constructs to us, and who recently passed away. We thank P. Kuhn and the SSRL staff for their assistance at beamlines 9-1 and 9-2 at the Stanford Synchrotron Radiation Laboratory and we thank M. Adler and J. Nicholson for the synaptobrevin peptide used in the co-crystal structure. We also greatly appreciate the neurotoxin provided by B. DasGupta, B. Tepp, E. Johnson, and M. Goodenough used in previous structural studies and their helpful discussions. Financial support for this research was provided by the U.S. Army and the NN20 program of the U.S. Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanson, M., Stevens, R. Cocrystal structure of synaptobrevin-II bound to botulinum neurotoxin type B at 2.0 Å resolution. Nat Struct Mol Biol 7, 687–692 (2000). https://doi.org/10.1038/77997
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/77997
- Springer Nature America, Inc.
This article is cited by
-
Low pH-Induced Pore Formation by the T Domain of Botulinum Toxin Type A is Dependent upon NaCl Concentration
The Journal of Membrane Biology (2010)
-
Mode of VAMP substrate recognition and inhibition of Clostridium botulinum neurotoxin F
Nature Structural & Molecular Biology (2009)
-
Retraction Note: Cocrystal structure of synaptobrevin-II bound to botulinum neurotoxin type B at 2.0 Å resolution
Nature Structural & Molecular Biology (2009)