Abstract
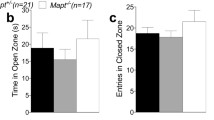
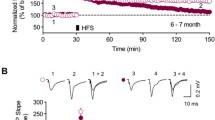
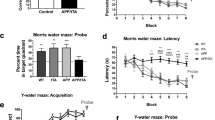
Proteolytic processing of amyloid precursor protein (APP) through an endosomal/lysosomal pathway generates carboxy-terminal polypeptides that contain an intact β-amyloid domain1–3. Cleavage by as-yet unidentified proteases releases the β -amyloid pep tide in soluble form4–6. In Alzheimer's disease, aggregated β-amyloid is deposited in extracellular neuritic plaques.Although most of the molecular mechanisms involvingβ-amyloid and APP in the aetiology of Alzheimer's disease are still unclear, changes in APP metabolism maybe important in the pathogenesis of the disease. Here we show that transgenic mice expressing the amyloidogenic carboxy-terminal 104 amino acids of APP develop, with ageing, extracellular β-amyloid immunoreactivity, increased gliosis and microglial reactivity, as well as cell loss hi the CA1 region of the hippocampus. Adult transgenic mice demonstrate spatial-learning deficits in the Morris water maze and in maintenance of long-term potentiation (LTP). Our results indicate that alterations in the processing of APP may have considerable physiological effects on synaptic plasticity.
Similar content being viewed by others
References
Estus, S. et al. Potentially amyloidogenic carboxyl-terminal derivatives of the amyloid protein precursor. Science 255, 726–728 (1992).
Haass, C., Koo, E. H., Mellon, A., Hung, A. Y. & Selkoe, D. J. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 357, 500–502 (1992).
Golde, T. E., Estus, S., Younkin, L. H., Selkoe, D. J. & Younkin, S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science 255, 728–730 (1992).
Haass, C. et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359, 322–325 (1992).
Shoji, M. et al. Production of the Alzheimer β-protein by normal proteolytic processing. Science 258, 126–129 (1992).
Seubert, P. et al. Isolation and quantification of soluble Alzheimer's β-peptide from biological fluids. Nature 359, 325–327 (1992).
Beaudet, L. et al. Intragenic regulatory elements contribute to transcriptional control of the neurofilament light gene. Gene 116, 205–214 (1992).
LeBlanc, A. Increased production of 4 kDa amyloid β-peptide in serum deprived human primary neuron cultures: possible involvement of apoptosis. J. Neurosci. 15, 7837–7846 (1995).
Meaney, M. J., Aitken, D. H., van Berkel, C., Bhatnagar, S. & Sapolsky, R. M. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 239, 766–768 (1988).
Morris, R. G. M. Development of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 11, 47–60 (1984).
Kolb, B., Sutherland, R. J. & Wishaw, I. Q. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behav. Neurosci. 97, 13–27 (1983).
Skelton, R. W. & McNamara, R. K. Bilateral knife cuts to the perforant path disrupt spatial learning in the Morris water maze. Hippocampus 2, 73–80 (1992).
Bliss, T. V. P. & Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Bear, M. F. & Malenka, R. C. Synaptic placticity: LTP and LTD. Curr. Opin. Neurobiol. 4, 389–399 (1994).
Yankner, B. A. et al. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science 245, 417–420 (1989).
Yoshikawa, K., Aizawa, T. & Hagashi, Y. Degeneration in vitro of post-mitotic neurons overexpressing the Alzheimer amyloid protein precursor. Nature 359, 64–67 (1992).
Hyman, B. T. Down syndrome and Alzheimer disease. Prog. Clin. Biol. Res. 379, 123–142 (1992).
Yankner, B. A., Duffy, L. K. & Kirschner, D. A. Neurotrophic and neurotoxic effects of amyloid β-protein: reversal by tachykinin neuropeptides. Science 250, 279–282 (1990).
Mattson, M. P. et al. β-amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 12, 376–389 (1992).
Loo, D. T. et al. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc. Natl Acad. Sti. USA 90, 7951–7955 (1993).
Behl, C., Davis, J. B., Lesley, R. & Schubert, D. Hydrogen peroxide mediates amyloid β-protein toxicity. Cell 77, 817–822 (1994).
Etcheberrigaray, R., Ito, E., Kim, C. S. & Alkin, D. L. Soluble β-amyloid induction of Alzheimer's phenotype of human fibroblast K+ channels. Science 264, 276–279 (1994).
Arispe, N., Rojas, J. & Pollard, H. B. Alzheimer disease amyloid β protein forms calcium channels in bilaycr membranes: blockade by trometbamine and aluminum. Proc. Natl Acad. Sci. USA 90, 567–571 (1993).
Meda, L. et al. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 374, 647–650 (1995).
Yan, S. D. et al. RAGE and amyloid β-peptide. Nature 382, 685–691 (1996).
El Khoury, J. et al. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature 382, 716–719 (1996).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice. Science 274, 99–103 (1996).
Games, D. et al. Alzheimer-type neuropathology in transgenic mice expressing V717F β-amyloid precursor. Nature 373, 523–526 (1995).
Quon, D. et al. Formation of β-amyloid protein deposits in brains of transgenic mice. Nature 352, 239–241 (1991).
Kang, J. et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325, 733–736 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nalbantoglu, J., Tirado-Santiago, G., Lahsaïni, A. et al. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature 387, 500–505 (1997). https://doi.org/10.1038/387500a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/387500a0
- Springer Nature Limited
This article is cited by
-
Accumulation of amyloid precursor protein C-terminal fragments triggers mitochondrial structure, function, and mitophagy defects in Alzheimer’s disease models and human brains
Acta Neuropathologica (2021)
-
Inhibition of Rac1-dependent forgetting alleviates memory deficits in animal models of Alzheimer’s disease
Protein & Cell (2019)
-
Not just amyloid: physiological functions of the amyloid precursor protein family
Nature Reviews Neuroscience (2017)
-
Pan-PPAR Modulation Effectively Protects APP/PS1 Mice from Amyloid Deposition and Cognitive Deficits
Molecular Neurobiology (2015)
-
The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes
Acta Neuropathologica (2015)