Abstract
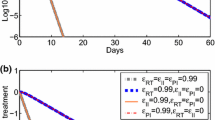
Analysis of changes in viral load after initiation of treatment with potent antiretroviral agents has provided substantial insight into the dynamics of human immunodeficiency virus type 1 (HIV-1)1–3. The concentration of HIV-1 in plasma drops by ∼99% in the first two weeks of treatment owing to the rapid elimination of free virus with a half-life (t1/2) of ≤6 hours and loss of productively infected cells with a t1/2 of 1.6 days3. Here we show that with combination therapy this initial decrease is followed by a slower second-phase decay of plasma viraemia. Detailed mathematical analysis shows that the loss of long-lived infected cells (t1/2of1–4weeks) is a major contributor to the second phase, whereas the activation of latently infected lymphocytes (t1/2 of 0.5–2 weeks) is only a minor source. Based on these decay characteristics, we estimate that 2.3–3.1 years of a completely inhibitory treatment would be required to eliminate HIV-1 from these compartments. To eradicate HIV-1 completely, even longer treatment may be needed because of the possible existence of undetected viral compartments or sanctuary sites.
Similar content being viewed by others
References
1. Wei,X.ef a/. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 273,117-122 (1995). 2. Ho, D. D. et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373, 123-126 (1995). 3. Perelson, A. S. et al. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271, 1582-1586 (1996). 4. Patick, A. K. et al. Antiviral and resistance studies of AG1343, an orally bioavailable inhibitor of human immunodeficiency virus protease. Antimicrob. Agents Chemother. 40, 292-297 (1996). 5. Heath, S. L. et al. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377, 740-744 (1995). 6. Pope, M. et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78, 389-398 (1994). 7. Haase, A. T. etal. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science 274,985-989(1996). 8. Herz, A. V. et al. Viral dynamics in vivo: limitations on estimates of intracellular delay and virus decay. Proc. NatlAcad. Sa. USA 93, 7247-7251 (1996). 9. Ho, D. D., Moudgil, T. & Alam, M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N. Engl. J. Med. 321, 1621-1625 (1989). 10. Connor, R. I., Mohri, H., Cao, Y. & Ho, D. D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. /. Virol. 67, 1772-1777 (1993). 11. Fazekas de St Groth, S. The evaluation of limiting dilution assays. /. Immunol. Meth. 49, Rl 1-R23 (1982). 12. Nowak, M. A., Bonhoeffer, S., Shaw, G. M. & May, R. M. Antiviral drug treatment: dynamics of resistance in free virus and infected cell populations. /. Theor. Biol. 184, 203-217 (1997). 13. Vesanen, M. et al. Stability in controlling viral replication identifies long-term nonprogressors as a distinct subgroup among human immunodeficiency virus type 1-infected persons. /. Virol. 70, 9035-9040 (1996). 14. Mitchie, C. A. et al. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature 360, 264-265 (1992). 15. Shaw, G. M. Viral and cellular dynamics in HIV-1 infection. Dixieme Colloque des Cent Gardes (eds Girard, M. & Dodet, B.) 9-11 (Elsevier, New York, 1995). 16. Chun, T.-W. etal. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nature Med. 1, 1284-1290 (1995). 17. Moreno, P. et al. Alveolar macrophages are not an important source of viral production in HIV-1 infected patients. AIDS 10, 682-684 (1996). 18. Koenig,. S. et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233, 1089-1093 (1986). 19. Cao, Y. et al. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS 6, 65-70(1992). 20. Ho, D. D., Rota, T. R., Hirsch, M. S. Infection of monocyte-macrophages by human T lymphotropic virus type III. /. Clin. Invest. 77, 1712-1715 (1986). 21. Gartner, S. etal. The role of mononuclear phagocytes in HTLV-II/LAV infection. Science 233,215-219 (1986). 22. Chun, T.-W. et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183-188 (1997). 23. Trepel, F. Number and distribution of lymphocytes in man. A critical analysis. Klin. Wschr. $2, 511-515(1974). 24. van Furth, R. Inflammation: Basic Principles and Clinical Correlates (eds Gallin, J. L, Goldstein, I. M. & Snyderman, S.) 325-339 (Raven, New York, 1992). 25. Efron, B. & Tibshirani, R. Bootstrap measures for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1, 54-77 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perelson, A., Essunger, P., Cao, Y. et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387, 188–191 (1997). https://doi.org/10.1038/387188a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/387188a0
- Springer Nature Limited
This article is cited by
-
Bispecific antibodies promote natural killer cell-mediated elimination of HIV-1 reservoir cells
Nature Immunology (2024)
-
Intestinal endothelial cells increase HIV infection and latency in resting and activated CD4 + T cells, particularly affecting CCR6 + CD4 + T cells
Retrovirology (2023)
-
Estimating the contribution of CD4 T cell subset proliferation and differentiation to HIV persistence
Nature Communications (2023)
-
Steering and controlling evolution — from bioengineering to fighting pathogens
Nature Reviews Genetics (2023)
-
Viral dynamics with immune responses: effects of distributed delays and Filippov antiretroviral therapy
Journal of Mathematical Biology (2023)