Abstract
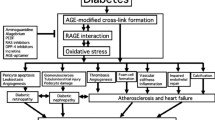
GLUCOSE and other reducing sugars react with proteins by a non-enzymatic, post-translational modification process called non-enzymatic glycosylation or glycation. The sugar-derived carbonyl group adds to a free amine, forming a reversible adduct which over time rearranges to produce a class of products termed advanced-glycation end-products (AGEs). These remain irreversibly bound to macromolecules and can covalently crosslink proximate amino groups1,2. The formation of AGEs on long-lived connective tissue and matrix components accounts largely for the increase in collagen crosslinking that accompanies normal ageing and which occurs at an accelerated rate in diabetes3,4. AGEs can activate cellular receptors and initiate a variety of pathophysiological responses5–9. They modify an appreciable fraction of circulating low-density lipoproteins preventing uptake of these particles by their high-affinity tissue receptors10,11. Advanced glycation has also been implicated in the pathology of Alzheimer's disease12,13. Because AGEs may form by a pathway involving reactive α-dicarbonyl intermediates1,2,14, we investigated a potential pharmacological strategy for selectively cleaving the resultant glucose-derived protein crosslinks. We now describe a prototypic AGE crosslink 'breaker', N-phenacylthiazolium bromide (PTB), which reacts with and cleaves covalent, AGE-derived protein crosslinks. The ability of PTB to break AGE crosslinks in vivo points to the importance of an α-dicarbonyl intermediate in the advanced glycation pathway and offers a potential therapeutic approach for the removal of established AGE crosslinks.
Similar content being viewed by others
References
Njoroge, F. G. & Monnier, V. M. Progr. clin. Biol. Res. 304, 85–107 (1989).
Bucala, R. & Cerami, A. Adv. Pharmac. 23, 1–34 (1992).
Monnier, V. M., Kohn, R. R. & Cerami, A. Proc. natn. Acad. Sci. U.S.A. 81, 583–587 (1984).
Monnier, V. M. et al. New Engl. J. Med. 314, 403–408 (1986).
Vlassara, H., Brownlee, M., Manogue, K. R., Dinarello, C. & Pasagian, A. Science 240, 1546–1548 (1988).
Esposito, C., Gerlach, H., Brett, J., Stern, D. & Vlassara, H. J. exp. Med. 174, 1387–1407 (1989).
Vlassara, H. et al. Proc. natn. Acad. Sci. U.S.A. 89, 12043–12047 (1992).
Doi, T. et al. Proc. natn. Acad. Sci. U.S.A. 89, 2873–2877 (1992).
Vlassara, V., Fuh, H., Donnelly, T. & Cybulsky, M. Molec. Med. 1, 447–456 (1995).
Bucala, R., Makita, Z., Koschinsky, T., Cerami, A. & Vlassara, H. Proc. natn. Acad. Sci. U.S.A. 90, 6434–6438 (1993).
Bucala, R. et al. Proc. natn. Acad. Sci. U.S.A. 91, 9441–9445 (1994).
Vitek, M. P. et al. Proc. natn. Acad. Sci. U.S.A. 91, 4766–4670 (1994).
Smith, M. A. et al. Proc. natn. Acad. Sci. U.S.A. 91, 5710–5714 (1994).
Chen, H.-J. & Cerami, A. J. Carbohydr. Chem. 12, 731–742 (1993).
Estendorfer, S., Ledl, F. & Severin, T. Angew. Chem. intl Ed. Engl. 29, 536–537 (1990).
Vovk, A. I., Murav'eva, I. V. & Yasnikov, A. A. Ukr. Khim. Zh. 51, 521 (1985).
Ledl, F. & Schleicher, E. Angew. Chem. 29, 565–593 (1990).
Eble, A. S., Thorpe, S. R. & Baynes, J. W. J. biol. Chem. 258, 9406–9412 (1983).
Makita, Z. et al. Science 258, 651–653 (1992).
Miyata, T. et al. J. clin. Invest. 92, 1243–1252 (1993).
Danze, P. M., Tarjoman, A., Rousseaux, J., Fossati, P. & Dautrevaux, M. Clin. chim. Acta. 166, 143–153 (1987).
Cotra, R. S., Kumar, V. & Robbins, S. L. Robbins Pathologic Basis of Disease 5th edn, 231–238 (Saunders, Philadelphia, 1994).
Ukai, O. K. et al. Yakugaku Zasshi 63, 296 (1943).
Makita, Z., Vlassara, H., Cerami, A. & Bucala, R. J. biol. Chem. 267, 5133–5138 (1992).
Bochantin, J. & Mays, L. L. Expl Gerontol. 16, 101–106 (1981).
Itakura, M. et al. Life Sci. 49, 889–897 (1991).
Stegeman, H. & Stadler, K. Clin. chim. Acta 18, 267–273 (1967).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasan, S., Zhang, X., Zhang, X. et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature 382, 275–278 (1996). https://doi.org/10.1038/382275a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/382275a0
- Springer Nature Limited
This article is cited by
-
Metabolic brain imaging with glucosamine CEST MRI: in vivo characterization and first insights
Scientific Reports (2023)
-
The role of advanced glycation end products in vascular aging: which parameter is the most suitable as a biomarker?
Journal of Human Hypertension (2021)
-
Serum biomarkers, skin autofluorescence and other methods. Which parameter better illustrates the relationship between advanced glycation end products and arterial stiffness in the general population?
Hypertension Research (2021)
-
Soluble receptor for advanced glycation end-products independently influences individual age-dependent increase of arterial stiffness
Hypertension Research (2020)
-
Association of physical fitness with skin autofluorescence-derived advanced glycation end products in children
Pediatric Research (2020)