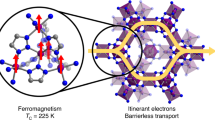
Abstract
THE rational design of molecular compounds that exhibit spontaneous magnetic ordering might enable one to tailor magnetic properties for specific applications in magnetic memory devices1–4. In such materials synthesized previously5–17, however, the underlying weak magnetic interactions are incapable of maintaining ordering at ambient temperatures. One remarkable exception is a compound derived from vanadium and tetracyanoethylene18, but the material is amorphous and fragile, and consequently the molecular interactions responsible for its striking properties are not understood. Here we demonstrate another route to the synthesis of a room-temperature organometallic magnet, in which we combine a hexa-cyanometalate [M(CN)6]q− with a Lewis acid Lp+ If L and M are transition-metal ions, then the orbital interactions in the resulting compound can be described by well understood principles21–24, and it is therefore possible to choose the metals to tune the compound's magnetic properties–in particular, the magnetic ordering (Curie) temperature Tc (refs 21–26). We have synthesized a room-temperature magnetic material (TC = 315 K) that belongs to the Prussian blue family of compounds27 (where M is chromium and L is vanadium), demonstrating that transition-metal hexacyano complexes are promising components for the construction of molecule-based high-Tc magnets.
Similar content being viewed by others
References
Miller, J. S. Angew. Chem. int. Edn Eng. 33, 385–415 (1994).
Gatteschi, D. Adv. Mater. 6, 635–645 (1994).
Verdaguer, M. et al. in Contributions to Development of Coordination Chemistry (eds Ondrejovic, G. & Sirota, A.) 19–24 (Slovak Tech. Univ. Press, Bratislava, 1993).
Kahn, O. Molecular Magnetism (VCH, New York, 1993).
Pei, Y., Verdaguer, M., Kahn, O., Sletten, J. & Renard, J. P. J. Am. chem. Soc. 108, 7428–7429 (1986).
Kahn, O., Pei, Y., Verdaguer, M., Renard, J. P. & Sletten, J. J. Am. chem. Soc. 110, 782–789 (1988).
Tamaki, H. et al. J. Am. chem. Soc. 114, 6974–6979 (1992).
Day, P. Accts chem. Res. 12, 236–243 (1979).
Descurtins, S. et al. Inorg. chim. Acta 216, 65–73 (1994).
Stumpf, H. O., Ouahab, L., Pei, Y., Grandjean, D. & Kahn, O. Science 261, 447–449 (1993).
Broderick, W. E., Thompson, J. A., Day, E. P. & Hoffman, B. M. Science 249, 401–403 (1990).
Caneschi, A., Gatteschi, D., Renard, J. P., Rey, P. & Sessoli, R. J. Am. chem. Soc. 111, 785–786 (1989).
Caneschi, A., Gatteschi, D., Sessoli, R. & Rey, P. Accts chem. Res. 22, 392–398 (1989).
Miller, J. S., Calabrese, J. C., McLean, R. S. & Epstein, A. J. Adv. Mater. 4, 298–300 (1992).
Nakazawa, Y. et al. Phys. Rev. B46, 8906–8914 (1992).
Allemand, P. M. et al. Science 253, 301–303 (1991).
Chiarelli, R., Nowak, M. A., Rassat, A. & Tholence, J. L. Nature 363, 147–149 (1993).
Manriquez, J. M., Yee, G. T., McLean, R. S., Epstein, A. J. & Miller, J. S. Science 252, 1415–1417 (1991).
Güdel, H. U., Stucki, H. & Lüdi, A. Inorg. chim. Acta 7, 121–124 (1973).
Lüdi, A. & Güdel, H. U. in Structure and Bonding Vol. 14 (eds Dunitz, J. D. et al.) 1–21 (Springer, Berlin, 1973).
Gadet, V., Mallah, T., Castro, I., Veillet, P. & Verdaguer, M. J. Am. chem. Soc. 114, 9213–9214 (1992).
Mallah, T., Thiébaut, S., Verdaguer, M. & Veillet, P. Science 262, 1554–1557 (1993).
Entley, W. R. & Girolami, G. S. Inorg. Chem. 33, 5165–5166 (1994).
Entley, W. R. & Girolami, G. S. Science 268, 397–402 (1995).
Bozorth, R. M., Williams, H. J. & Walsh, D. E. Phys. Rev. 103, 572–578 (1956).
Babel, D. Comments inorg. Chem. 5, 285–320 (1982).
Anonymous, Miscellanea berolinensia ad Incrementum scientiarum Vol. l, 377–378 (Berlin, 1710).
Nakamoto, K. Infra-red and Raman Spectra of Inorganic and Coordination Compounds 259–267 (Wiley, New York, 1978).
Wong, J., Lytle, F., Messmer, R. P. & Maylotte, D. H. Phys. Rev. B30, 5596–5610 (1984).
Néel, L. Annls Phys. 3, 137–198 (1948).
Köningsberger, D. C. & Prins, R. (eds) X-ray Absorption (Wiley, New York, 1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferlay, S., Mallah, T., Ouahès, R. et al. A room-temperature organometallic magnet based on Prussian blue. Nature 378, 701–703 (1995). https://doi.org/10.1038/378701a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/378701a0
- Springer Nature Limited
This article is cited by
-
Exploration of glassy state in Prussian blue analogues
Nature Communications (2022)
-
Random transverse single-ion anisotropies in the mixed spin-1 and spin-1/2 Blume–Capel quantum model: Mean-field theory calculations
Pramana (2022)
-
Charge transfer driven by ultrafast spin transition in a CoFe Prussian blue analogue
Nature Chemistry (2021)
-
Proton switching molecular magnetoelectricity
Nature Communications (2021)
-
Magnetic ordering through itinerant ferromagnetism in a metal–organic framework
Nature Chemistry (2021)