Abstract
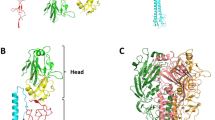
HAEMAGGLUTININ (HA) is the influenza surface glycoprotein that interacts with infectivity-neutralizing antibodies. As a consequence of this immune pressure, it is the variable virus component, which is important in antigenic drift, that results in recurrent epidemics of influenza. We have determined the crystallographic structure of a complex formed between the antigen-binding fragment (Fab) of a neutralizing antibody and the membrane-distal domain ((HA top9) of a HA subunit prepared from HA in its membrane-fusion-active conformation. A dramatic change is seen in the structure of the Fab-combining site on complex formation. Our results indicate that neutralization of infectivity by this antibody involves the inhibition of receptor binding, and demonstrate how influenza virus can maintain its conserved receptor-binding site despite the immune selective pressure for change in this region of the molecule; they also contribute to a complete description of the endosomal pH-induced fusion-active HA structure.
Similar content being viewed by others
References
Wilson, I. A., Skehel, J. J. & Wiley, D. C. Nature 289, 366–373 (1981).
Wiley, D. C. & Skehel, J. J. A. Rev. Biochem. 56, 365–394 (1987).
Weis, W. et al. Nature 333, 426–431 (1988).
Bullough, P. A., Hughson, F. M., Skehel, J. J. & Wiley, D. C. Nature 371, 37–43 (1994).
Ruigrok, R. W., Aitken, A., Calder, L. J. & Markin, S. R. J. gen. Virol., 2785–2795 (1988).
Wharton, S. A. et al. J. biol. Chem. 263, 4474–4480 (1988).
Daniels, R. S., Douglas, A. R., Skehel, J. J. & Wiley, D. C. J. gen. Virol. 64, 1657–1662 (1983).
Daniels, R. S. et al. Cell 40, 431–439 (1985).
Godley, L. et al. Cell 68, 635–645 (1992).
Ruigrok, R. W. et al. EMBO J. 5, 41–49 (1986).
Bizebard, T. et al. Acta crystallogr. D50, 768–777 (1994).
Wrigley, N. G. et al. Virology, 308–314 (1983).
Wilson, I. A. & Stanfield, R. L. Curr. Opin. struct. Biol. 4, 857–867 (1994).
Cherfils, J., Bizebard, T., Knossow, M. & Janin, J. Proteins 18, 8–18 (1994).
Janin, J. & Chothia, C. J. biol. Chem. 265, 16027–16030 (1990).
Wilson, I. A. & Cox, N. J. A. Rev. Immun. 8, 737–771 (1990).
Knossow, M., Daniels, R. S., Douglas, A. R., Skehel, J. J. & Wiley, D. C. Nature 311, 678–680 (1984).
Parry, N. et al. Nature 347, 569–572 (1990).
Skehel, J. J. & Schild, G. C. Virology 44, 396–408 (1971).
Bizebard, T. et al. J. molec. Biol. 216, 513–514 (1990).
Leslie, A. G. W. CCP4 and ESF-EACMB Newsl. Prot. Crystallogr. no. 26 (SERC, Daresbury Lab., Warrington, 1992).
Collaborative Computing Project no. 4 Acta crystallogr. D50, 760–763 (1994).
Navaza, J. Acta Crystallogr. A50, 157–163 (1994).
Jones, T. A. Meth. Enzym. 115, 157–171 (1985).
Brünger, A. T. X-PLOR (version 3.1) Manual (Yale Univ., New Haven, 1992).
Laskowski, R. A., McArthur, M. W., Moss, D. S. & Thornton, J. M. J. appl. Crystallogr. 26, 283–291 (1993).
Shrake, A. & Rupley, J. A. J. molec. Biol. 79, 351–371 (1973).
Ward, C. W. Curr. Top. Microbiol. Immun. 94/95, 1–74 (1981).
Daniels, R. S. et al. EMBO J. 6, 1459–1465 (1987).
Kraulis, P. J. appl. Crystallogr. 24, 924–950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bizebard, T., Gigant, B., Rigolet, P. et al. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature 376, 92–94 (1995). https://doi.org/10.1038/376092a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/376092a0
- Springer Nature Limited
This article is cited by
-
The shape of pleomorphic virions determines resistance to cell-entry pressure
Nature Microbiology (2021)
-
The evolution of seasonal influenza viruses
Nature Reviews Microbiology (2018)
-
AS03-adjuvanted H5N1 vaccine promotes antibody diversity and affinity maturation, NAI titers, cross-clade H5N1 neutralization, but not H1N1 cross-subtype neutralization
npj Vaccines (2018)
-
Receptor mimicry by antibody F045–092 facilitates universal binding to the H3 subtype of influenza virus
Nature Communications (2014)
-
Changed epitopes drive the antigenic drift for influenza A (H3N2) viruses
BMC Bioinformatics (2011)