Abstract
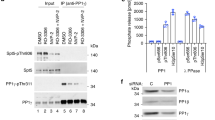
THE RNA polymerase II holoenzyme consists of RNA polymerase II, a subset of general transcription factors, and regulatory proteins known as SRB proteins1,2. The genes encoding SRB proteins were isolated as suppressors of mutations in the RNA polymerase II carboxy-terminal domain (CTD)3,4. The CTD and SRB proteins have been implicated in the response to transcriptional regulators1–11. We report here the isolation of two new SRB genes, SRB10 and SRB11, which encode kinase- and cyclin-like proteins, respectively. Genetic and biochemical evidence indicates that the SRB 10 and SRB 11 proteins form a kinase–cyclin pair in the holoenzyme. The SRB10/11 kinase is essential for a normal transcriptional response to galactose induction in vivo. Holoenzymes lacking SRB 10/11 kinase function are strikingly deficient in CTD phosphory lation. Although defects in the kinase substantially affect transcription in vivo, purified holoenzymes lacking SRB10/11 kinase function do not show defects in definedin vitro transcription systems, suggesting that the factors necessary to elicit the regulatory role of the SRB10/11 kinase are missing in these systems. These results indicate that the SRB 10/11 kinase is involved in CTD phosphorv lation and suggest that this modification has a role in the response to transcriptional regulators in vivo.
Similar content being viewed by others
References
Koleske, A. J. & Young, R. A. Nature 368, 466–469 (1994).
Kim, Y.-J., Bjorklund, S., Li, Y., Sayre, M. H. & Kornberg, R. D. Cell 77, 599–609 (1994).
Koleske, A. J., Buratowski, S., Nonet, M. & Young, R. A. Cell 69, 883–894 (1992).
Thompson, C. M., Koleske, A. J., Chao, D. M. & Young, R. A. Cell 73, 1361–1375 (1993).
Allison, L. A. & Ingles, C. J. Proc. natn. Acad. Sci. U.S.A. 86, 2794–2798 (1989).
Scafe, C. et al. Nature 347, 491–494 (1990).
Peterson, C. L., Kruger, W. & Herskowitz, I. Cell 64, 1135–1143 (1991).
Liao, S.-M., Taylor, I. C. A., Kingston, R. E. & Young, R. A. Genes Dev. 5, 2431–2440 (1991).
Li, Y. & Kornberg, R. D. Proc. natn. Acad. Sci. U.S.A. 91, 2362–2366 (1994).
Young, R. A. Rev. Biochem. 60, 689–715 (1991).
Dahmus, M. E. & Dynan, W. S. in Transcriptional Regulation 109–128 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1992).
Lorincz, A. T. & Reed, S. I. Nature 307, 183–185 (1984).
Surosky, R. T., Strich, R. & Esposito, R. E. Molec. cell. Biol. 13, 3446–3458 (1994).
Uesono, Y., Tanka, K. & Toh-e, A. Nucleic Acids Res. 15, 10299–10309 (1987).
Lees, J. M. & Greenleaf, A. L. Gene Expr. 1, 149–167 (1991).
Simon, M., Seraphin, B. & Faye, G. EMBO J. 5, 2697–2701 (1986).
Lew, D. J., Dulic, V. & Reed, S. I. Cell 66, 1197–1206 (1991).
Leopold, P. & O'Farrell, P. H. Cell 66, 1207–1216 (1991).
Xiong, Y., Menninger, J., Beach, D. & Ward, D. C. Genomics 13, 575–584 (1992).
Kobayashi, H. et al. Molec. Biol. Cell 3, 1279–1294 (1992).
Lees, E. M. & Harlow, E. Molec. cell. Biol. 13, 1194–1201 (1993).
Fields, S. & Song, O. Nature 340, 245–246 (1989).
O'Brien, T., Hardin, S., Greenleaf, A. & Lis, J. T. Nature 370, 75–77 (1994).
Feaver, W. J., Svejstrup, J. Q., Henry, N. L. & Kornberg, R. D. Cell 79, 1103–1110 (1994).
Feaver, W. J., Gileadi, O., Li, Y. & Kornberg, R. Cell 67, 1223–1330 (1991).
Kolodziej, P. & Young, R. A. Meth. Enzym. 194, 508–519 (1991).
Smith, D. B. & Johnson, K. S. Gene 67, 31–40 (1988).
Rothstein, R. Meth. Enzyme. 194, 281–301 (1991).
Sayre, M. H., Tschochner, H. & Kornberg, R. D. J. biol. Chem. 267, 23383–23387 (1992).
Kim, T. K. et al. Nature 369, 252–255 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liao, SM., Zhang, J., Jeffery, D. et al. A kinase–cyclin pair in the RNA polymerase II holoenzyme. Nature 374, 193–196 (1995). https://doi.org/10.1038/374193a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/374193a0
- Springer Nature Limited
This article is cited by
-
Transcriptional regulatory proteins in central carbon metabolism of Pichia pastoris and Saccharomyces cerevisiae
Applied Microbiology and Biotechnology (2020)
-
Kin28 regulates the transient association of Mediator with core promoters
Nature Structural & Molecular Biology (2014)
-
Cyclin C is a haploinsufficient tumour suppressor
Nature Cell Biology (2014)
-
A conserved Mediator–CDK8 kinase module association regulates Mediator–RNA polymerase II interaction
Nature Structural & Molecular Biology (2013)
-
Structure and Basal Transcription Complex of RNA Polymerase II Core Promoters in the Mammalian Genome: An Overview
Molecular Biotechnology (2010)