Abstract
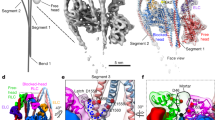
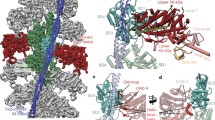
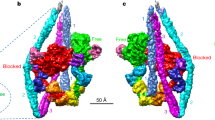
The regulatory domain of scallop myosin is a three-chain protein complex that switches on this motor in response to Ca2+ binding. This domain has been crystallized and the structure solved to 2.8 Å resolution. Side-chain interactions link the two light chains in tandem to adjacent segments of the heavy chain bearing the IQ-sequence motif. The Ca2+-binding site is a novel EF-hand motif on the essential light chain and is stabilized by linkages involving the heavy chain and both light chains, accounting for the requirement of all three chains for Ca2+ binding and regulation in the intact myosin molecule.
Similar content being viewed by others
References
Kendrick-Jones, J., Lehman, W. & Szent-Györgyi, A. G. J. molec. Biol. 54, 313–326 (1970).
Szent-Györgyi, A. G., Szentkiralyi, E. M. & Kendrick-Jones, J. J. molec. biol. 74, 179–203 (1973).
Wells, C. & Bagshaw, C. R. Nature 313, 696–697 (1985).
Chantler, P. D. & Szent-Györgyi, A. G. J. molec. Biol. 138, 473–492 (1980).
Wallimann, T. Szent-Györgyi, A. G. Biochemistry 20, 1176–1187 (1981).
Kwon, H. et al. Proc. natn. Acad. Sci. U.S.A. 87, 4771–4775 (1990).
Szentkiralyi, E. M. J. Muscle Res. Cell Motil. 5, 147–164 (1984).
Fromherz, S. & Szent-Györgyi, A. G. Biophys. J. 64a, 7 (1993).
Rayment, I. et al. Science 261, 50–58 (1993).
Ikura, M. et al. Science 256, 632–638 (1992).
Meador, W. E., Means, A. R. & Quiocho, F. A. Science 257, 1251–1255 (1992).
Mercer, J. A., Seperack, P. K., Strobel M. C., Copeland, N. G. & Jenkins, N. A. Nature 349, 709–713 (1991).
Cheney, R. J. & Mooseker, M. S. Curr. Opin. Cell Biol. 4, 27–35 (1992).
Persechini, A. & Kretsinger, R. H. J. biol. chem. 263, 12175–17178 (1988).
Collins, J. H. J. Muscle Res. Cell Motil. 12, 3–25 (1991).
Kretsinger, R. H. & Nockolds, C. E. J. biol. Chem. 248, 3313–3326 (1973).
Bennett, A. G. & Bagshaw, C. R. Biochem. J. 233, 173–179 (1986).
da Silva, A. C. R. & Reinach, F. C. Trends biochem. Sci. 16, 53–57 (1991).
Strynadka, N. C. J. & James, M. N. Curr. Opin. struct. Biol. 1, 905–914 (1991).
Szent-Györgyi, A. G. & Chantler, P. D. in Myology Vol. 2 (eds Engel, A. G. & Franzini-Armstrong, C.) 506–528 (McGraw-Hill, New York, 1994).
Sellers, J. R., Chantler, P. D. & Szent-Györgyi, A. G. J. Molec. Biol. 144, 223–245 (1980).
Rowe, T. & Kendrick-Jones, J. EMB0 J. 11, 4715–4722 (1992).
Hardwicke, P. M. D., Wallimann, T. & Szent-Györgyi, A. G. Nature 301, 478–482 (1983).
Trybus, K. M. & Chatman, T. A. J. biol. Chem. 268, 4412–4419 (1993).
Vibert, P. & Cohen, C. J. Musc. Res. Cell Motility 9, 296–305 (1988).
Rayment, I. et al. Science 261, 58–65 (1993).
Wells, J. A. & Yount, R. G. Meth. Enzym. 85, 93–116 (1982).
Berchtold, H. et al. Nature 365, 126–132 (1993).
Kjeldgaard, M., Nissen, P., Thirup, S. & Nyborg, J. Structure 1, 35–50 (1993).
Wang, B.-C. Meth. Enzym. 115, 90–112 (1985).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Acta crystallogr. A47, 110–119 (1991).
Kabsch, W., Mannherz, H. G., Suck, D., Pai, E. F. & Holmes, K. C. Nature 347, 37–44 (1990).
Brünger, A. T. X-PLOR v. 3.0 Manual (Yale Univ., New Haven, 1992).
Stafford, W. F., Szentkiralyi, E. M. & Szent-Györgyi, A. G. Biochemistry 24, 5273–5280 (1979).
Funk, M. O., Nakagawa, Y., Skochdopole, J. & Kaiser, E. T. Int. J. Pept. Prot. Res. 13, 296–303 (1979).
Kraulis, P. J. J. appl. Crystallogr. 24, 946–950 (1991).
Sekharudu, C. Y. & Sundaralingam, M. Prot. Sci. 2, 620–625 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xie, X., Harrison, D., Schlichting, I. et al. Structure of the regulatory domain of scallop myosin at 2.8 Ä resolution. Nature 368, 306–312 (1994). https://doi.org/10.1038/368306a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/368306a0
- Springer Nature Limited
This article is cited by
-
Smooth muscle-like Ca2+-regulation of actin–myosin interaction in adult jellyfish striated muscle
Scientific Reports (2018)
-
Molecular basis of AKAP79 regulation by calmodulin
Nature Communications (2017)
-
Molecular mechanisms of cardiomyopathy phenotypes associated with myosin light chain mutations
Journal of Muscle Research and Cell Motility (2015)
-
Analysis of tarantula skeletal muscle protein sequences and identification of transcriptional isoforms
BMC Genomics (2009)
-
Gene expression analyses of essential catch factors in the smooth and striated adductor muscles of larval, juvenile and adult great scallop (Pecten maximus)
Journal of Muscle Research and Cell Motility (2009)